On this page
These guidelines were published in 2019 and are awaiting review, due 2022. Some content may be outdated.
The most common cardiac anomalies are summarised in this section.
The main reference source for this section is A Practical Guide to Fetal Echocardiography: Normal and abnormal hearts (Abuhamad and Chaoui 2015).
For more detail, please refer to this guide or another fetal echocardiography text.
Ventricular septal defects
- VSDs are the most commonly prenatally-detected cardiac anomaly.
- A VSD is an opening in the ventricular septum, leading to a shunt between the two ventricles.
- VSDs (particularly perimembranous) are frequently associated with other cardiac anomalies, particularly conotruncal anomalies. Identification of a VSD should prompt careful review of the heart.
- If isolated, the majority close within the first year of life.
VSDs are usually classified by their location. The most common VSDs identified prenatally are muscular and perimembranous.
VSDs may also be outlet or inlet in location. For more information, see Appendix 8: Anatomic locations of ventricular septal defects.
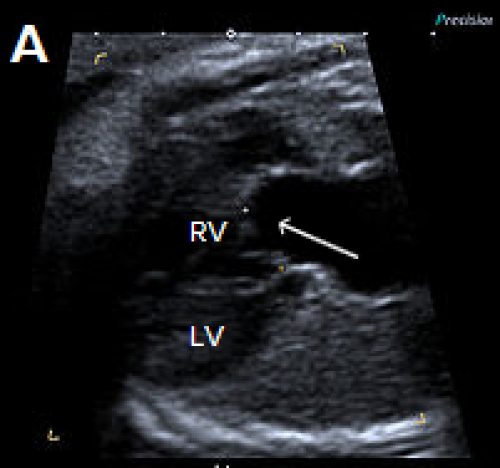
1. Perimembranous VSD
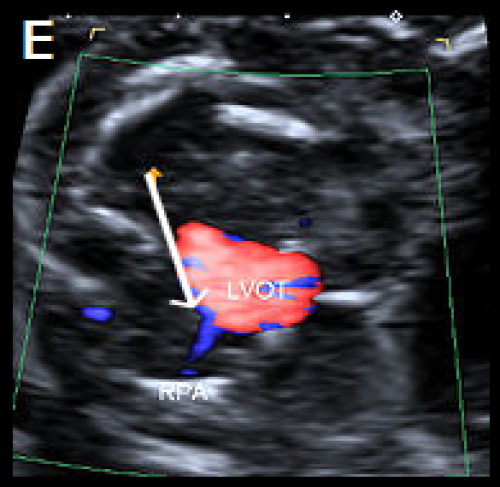
- Perimembranous VSDs are located in the outflow tract beneath the aortic valve.
- They are the most common type of VSD postnatally but may be more difficult to detect prenatally than muscular VSD.
- On greyscale imaging, the VSD is best visualised in the LVOT view, with loss of continuity of the ventricular septum and aorta.
- Colour Doppler may identify VSDs not visualised on 2D imaging.
- Perimembranous VSD can be associated with chromosome abnormality and requires Fetal Medicine review.
Image 12
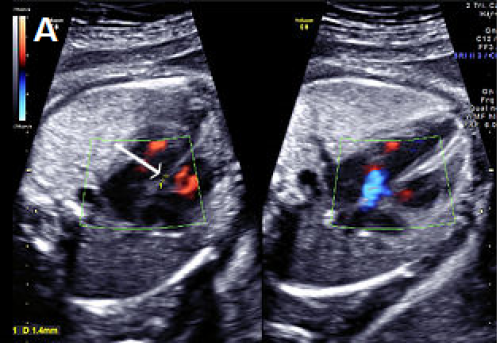

Small perimembranous VSD (arrow) on greyscale and colour Doppler imaging.
Image 13
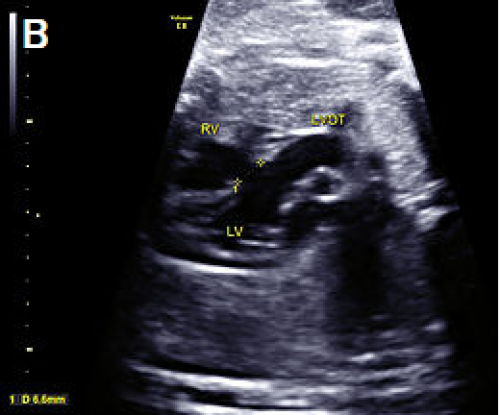

Larger VSD in the LVOT view, shown as discontinuity of the ventricular septum and aorta.
Image 14
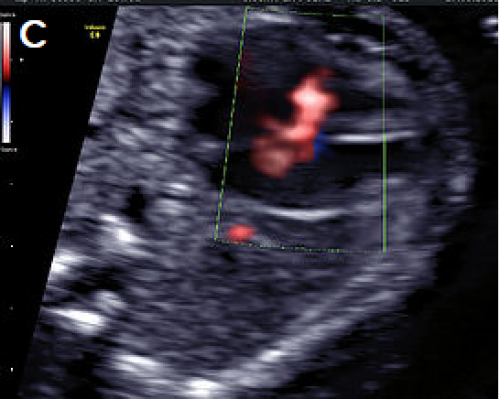

Perimembranous VSD on colour Doppler imaging.
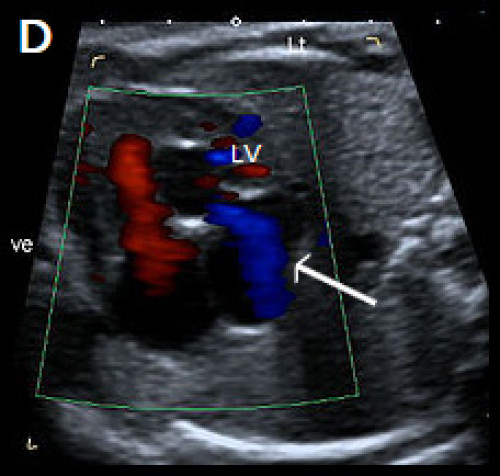
2. Muscular VSD
- Located in the muscular septum; may be mid-muscular, apical or multiple (‘Swiss cheese’ septum)
- Account for 10–15 percent of VSDs, but muscular VSDs are the most commonly-detected VSD prenatally
- Frequently close spontaneously
- Approximately 3 percent recurrence risk to siblings
- Rarely visualised on greyscale imaging unless large (>2–3 mm)
- Best identified in the apical or transverse 4Ch view
- The borders of the VSD often appear echogenic (unlike the dropout artefact commonly seen on the apex-up 4Ch view)
- Most easily visualised with colour Doppler, with bi-directional shunt in most cases
- Most common location is the apex or mid septum
- If isolated, muscular VSD is usually benign and not associated with chromosomal anomaly.
Image 15
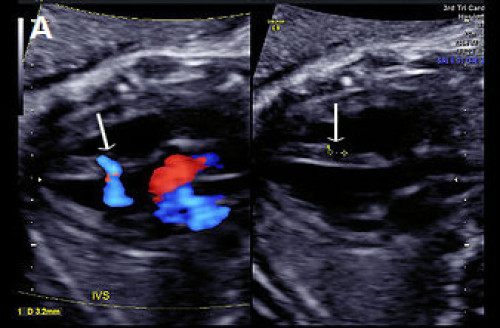

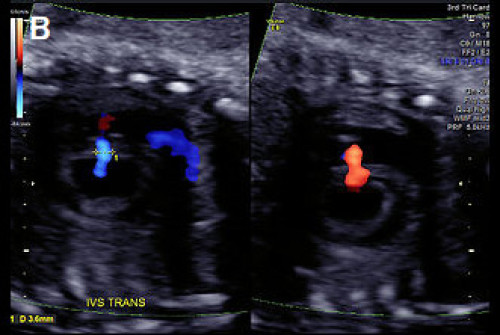

(A) Muscular VSD (arrow) on colour Doppler and greyscale imaging in the transverse long-axis IVS plane (arrows) and (B) in the transverse short-axis IVS plane with colour Doppler, showing bi‑directional flow.
Atrioventricular septal defect
- Characterised by a deficient AV septum and abnormalities of the AV valves, usually a common AV junction
- Also known as AV canal defect or endocardial cushion defect
- Relatively common cardiac defect (approximately 5–7 percent of congenital cardiac anomalies)
- Associations:
- Other cardiac anomalies, particularly conotruncal abnormalities
- Chromosomal anomaly, particularly trisomy 21.
AVSD may be complete or partial.
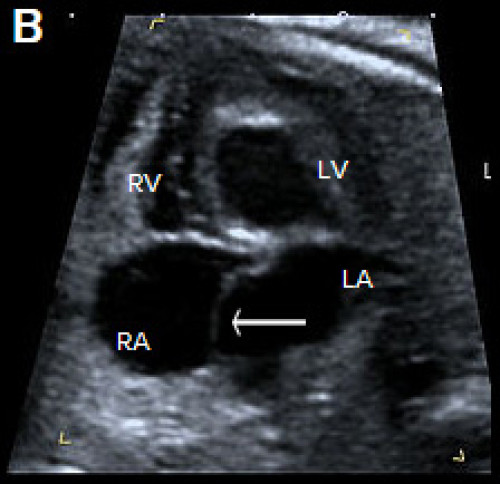
- Complete – Combination of atrial septum primum defect and inlet VSD with an abnormal common AV valve connecting to both ventricles. On ultrasound, this appears as a large central cardiac defect in diastole (when the valve is open), with blood flow between all four chambers and a common AV valve. In systole (when the valve is closed), there is loss of the normal apical offset of the tricuspid valve insertion on the septum. The common valve appears as a curvilinear continuous echogenic line. The ventricular size can be assessed for an unbalanced AVSD.
- Partial – Atrial septum primum defect with both mitral and tricuspid valve annuli usually present but loss of the normal offsetting of the AV valves, which attach at the same level on the IVS (rather than more the apical offset of the tricuspid valve observed in the normal heart).
Ultrasound features of partial AVSD are a linear AV valve insertion and atrial septum primum defect, but without a large VSD.
AVSD may be balanced or unbalanced (where the AV connection drains predominantly to one ventricle, causing disproportion in ventricular size).
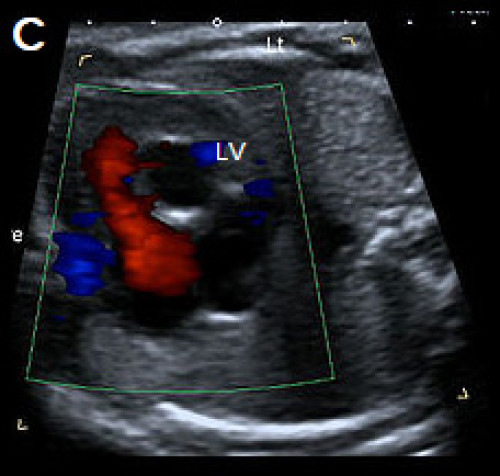
Colour Doppler is helpful in confirming the diagnosis, showing a single channel of blood flow to the ventricles, dividing over the remaining ventricular septum, and common valve regurgitation in most cases of complete AVSD.
Image 16
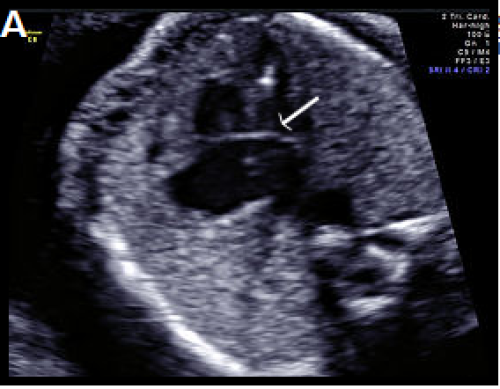

Complete AVSD with common AV valve (arrow) in systole (valve closed).
Image 17
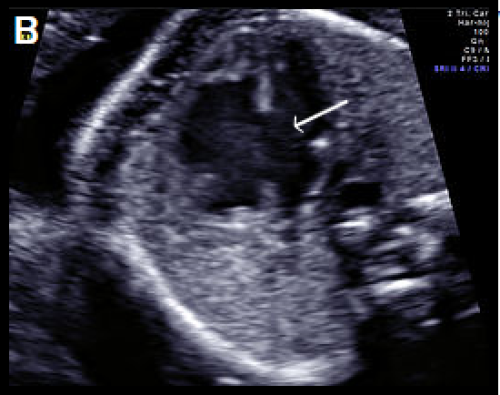

Complete AVSD with common AV valve in diastole (valve open) with a large central defect (arrow).
Image 18
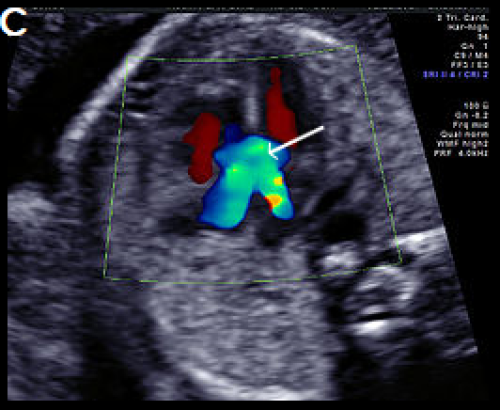

Complete AVSD with colour Doppler filling the large central defect and common valve regurgitation (arrow).
Other atrial septal defects
- Atrial septal defects (ASDs) are rarely detected prenatally and often not detectable until birth.
- Defined as an abnormal opening of the atrial septum, with communication between the left and right atria.
- Common: in approximately 7 percent of infants with congenital cardiac abnormalities.
- Incidence: about 1:1,500 live births.
- When detected prenatally, careful cardiac review for associated anomalies is required (including AVSD, isomerism, anomalous pulmonary venous drainage and aortic coarctation).
- Beware diagnosing an ASD when a persistent left SVC is present. Assess with colour Doppler for left-to-right shunting due to a dilated coronary sinus.
- In the third trimester, the foramen ovale flap may appear redundant (‘aneurysm of the foramen ovale’), a normal variant that should not be confused with an ASD.
Classified according to embryonic origin and location as:
- Septum primum ASD (ASD I)
- Septum secundum ASD (ASD II)
- Sinus venosus ASD – rare and difficult to detect prenatally
- Coronary sinus defect – rare and difficult to detect prenatally.
1. Septum primum (ASD I)
- Also known as partial AVSD.
- Second most common type of ASD (after secundum).
- Characterised by a gap in the embryologic septum primum, adjacent to both AV valves.
- Commonly associated with aneuploidies, such as trisomy 21.
- Can be detected prenatally as a gap in the septum primum, often associated with lack of the normal offsetting (linear insertion) of the AV valves (as seen with an AVSD, see above).
- Colour Doppler may confirm the ASD by demonstrating right-to-left shunting of blood across the atrial septum adjacent to the AV valves and separate from the normal foramen ovale.
- Beware: The normal or dilated coronary sinus may be mistaken for a septum primum ASD but shows colour Doppler flow from left to right into the right atrium.
Image 19
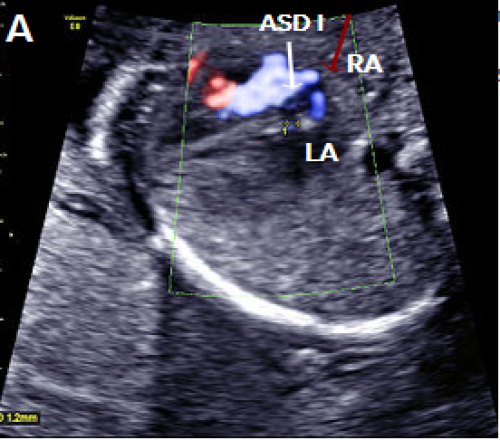

Septum primum ASD (white arrow) shown as a gap in the septum primum separate from the normal foramen ovale with right-to-left flow in blue (red arrow).
Image 20
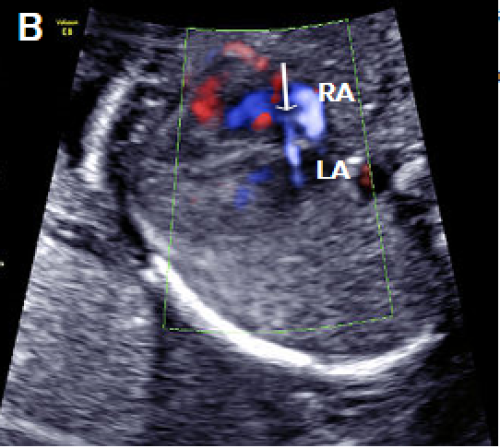

Septum primum ASD (white arrow), with right-to-left flow across the atrial septum (blue).
Image 21
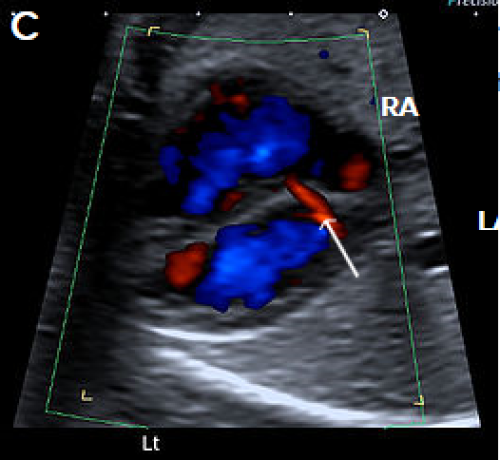

Normal coronary sinus (arrow) with left-to-right flow (red), which should not be misinterpreted as an ASD.
2. Septum secundum ASD (ASD II)
- Most common: approximately 80 percent of all ASDs but very difficult to identify prenatally.
- Characterised by a lack of tissue in the region of the foramen ovale.
- Associated with partial anomalous venous drainage (in 10–15 percent of cases).
- Beware: The redundant foramen ovale flap (‘aneurysm of the foramen ovale’) is a normal variant of the foramen ovale seen most commonly in the third trimester and should not be mistaken for an ASD.
Tetralogy of Fallot
Tetralogy of Fallot (TOF) is one of the most common forms of cyanotic heart disease, occurring in about 1:3,500 live births.
TOF accounts for approximately 5 percent of cases of congenital heart disease.
TOF has three major prenatal components. These are:
- malaligned (subaortic) VSD
- overriding aorta (aortic root overrides the VSD)
- pulmonary stenosis.
The fourth component of the TOF is right ventricular hypertrophy and is usually not identified prenatally.
Ultrasound findings
- The 4Ch view will generally appear normal, unless the VSD is large.
- TOF is best identified in the LVOT view as a perimembranous subaortic VSD and overriding aortic root, with discontinuity between the IVS and medial aortic wall (malalignment VSD). The aortic root is slightly aligned to the right, overriding the right ventricle (aortic dextroposition), and is often dilated.
- Pulmonary stenosis is best identified in the 3-vessel or short axis view and may be subtle, particularly in the second trimester. More severe forms are TOF with pulmonary atresia or absent pulmonary valve.
- Doppler is useful in confirming the diagnosis, demonstrating the VSD shunt and the overriding aorta with flow from both ventricles into the aortic root (Y-sign).
- Flow across the ductus arteriosus is antegrade in milder forms of TOF but may be reversed in severe cases.
- Measure branch pulmonary artery calibre.
- Assess for presence of the thymus and right-sided aortic arch – thymic hypoplasia and RAA are associated with DiGeorge syndrome (22q11.2 deletion).
Associations
- Other cardiac abnormalities, such as right-sided aortic arch, aberrant origin of a subclavian artery, AVSD, patent foramen ovale, ASD, persistent left SVC
- Extra-cardiac abnormalities
- Chromosomal abnormality in about 30 percent (particularly when associated with an AVSD) – mainly trisomies 21, 13 and 18, 22q11.2 deletion (10–15 percent)
- Genetic syndromes
- Increased NT.
Image 22

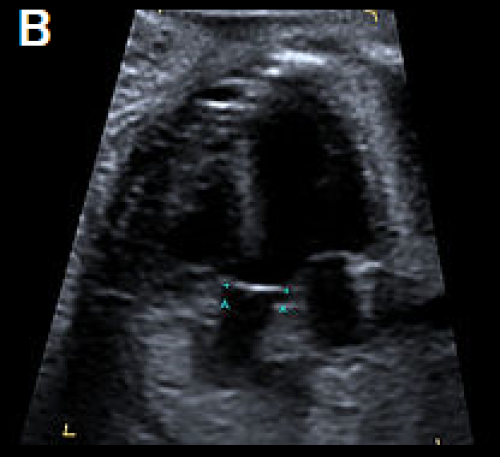

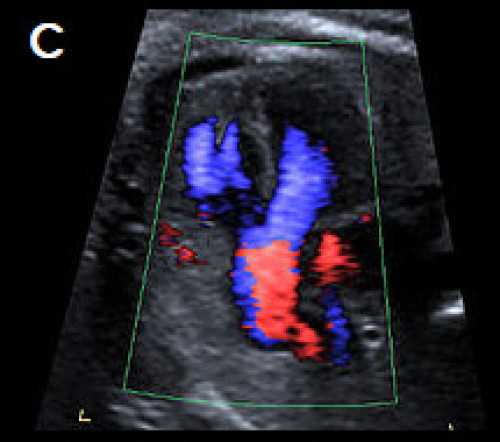

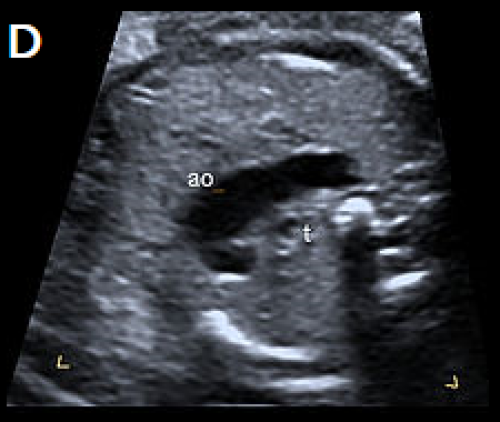

4Ch view (apex up) showing perimembranous VSD (A). Overriding, dilated aortic root with perimembranous VSD (B) and Y-sign with colour Doppler (blood from both ventricles flowing into the aortic root) on apex-up LVOT view in the same fetus (C).
On the 3-vessel view only a single large vessel (the aorta) is evident in this case (D); colour Doppler may aid in demonstrating a small pulmonary artery.
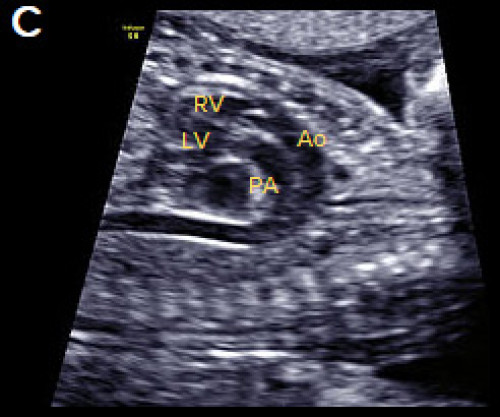
Transposition of the great arteries
1. Complete transposition of the great arteries
- Complete transposition of the great arteries (D-TGA) is characterised by AV concordance (normally connected atria and ventricles, that is, right atrium to right ventricle, RV, and left atrium to left ventricle, LV).
- Ventriculoarterial discordance (switched connections of the great vessels – the PA arises from the LV and the aorta arises from the RV).
- Relatively common, accounting for approximately 5 percent of congenital cardiac anomalies.
Ultrasound findings
- The 4Ch view is usually normal.
- There is a parallel course of the great vessels, rather than crossing as in the normal situation.
- The aorta is located anterior and to the right of the PA (hence D-TGA) and runs parallel to the PA.
- On the LVOT view, the PA arises from the LV and bifurcates shortly after its origin into the branch PAs.
- The normal 3-vessel view cannot be obtained – instead a single large vessel (the transverse aortic arch) is visualised, with the SVC located to its right.
- In the short-axis plane of the great vessels, the PA and aorta are adjacent to each other, rather than the longitudinal PA wrapping around the circular aortic root.
- In the longitudinal plane, the aortic arch, giving rise to the head and neck vessels, arises from the anterior-most RV.
- Colour Doppler may help in confirming the diagnosis, and in assessing for associated anomalies, particularly in early gestation.
Associations
- Other cardiac anomalies, for example, VSD, particularly perimembranous (40 percent of cases), and pulmonary stenosis (most commonly detected in the third trimester).
- D-TGA is often an isolated anomaly, and extra-cardiac anomalies are uncommon.
- Microdeletion of 22q11.2 may be associated with complex D-TGA, especially when there are associated extra-cardiac anomalies.
- Abnormal situs, for example, abdominal situs inversus, which may allow a balanced circulation if there are associated veno-atrial connection anomalies.
Image 23
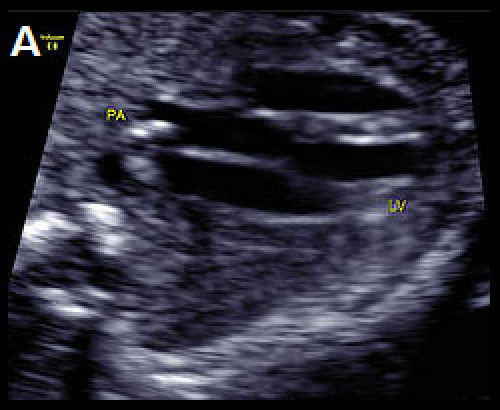

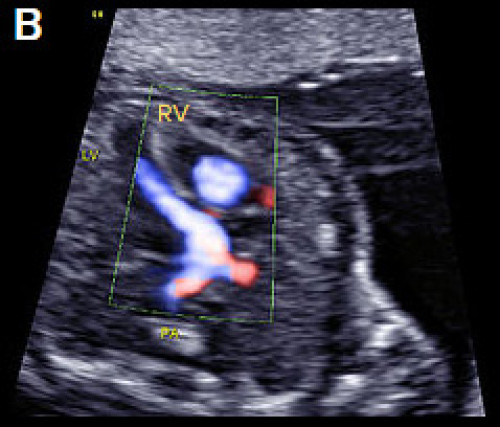

D-TGA with ventriculoarterial discordance. The bifurcating PA arises from the LV on the transverse long-axis LVOT plane, in B‑mode (A) and colour Doppler imaging, apex-up (B).
Image 24

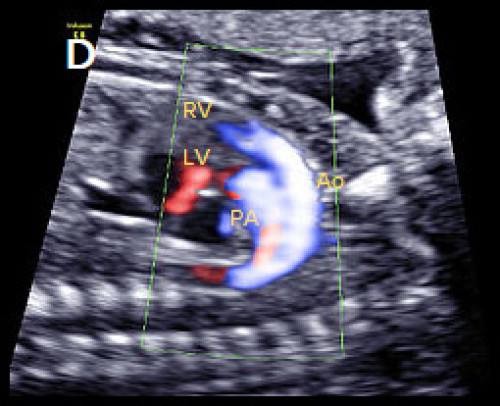

In the longitudinal plane, the aorta (Ao) with head and neck vessels arising from it, arises from the anterior RV, and the PA arises posteriorly from the posterior LV, on B-mode (C) and colour Doppler (D).
Image 25
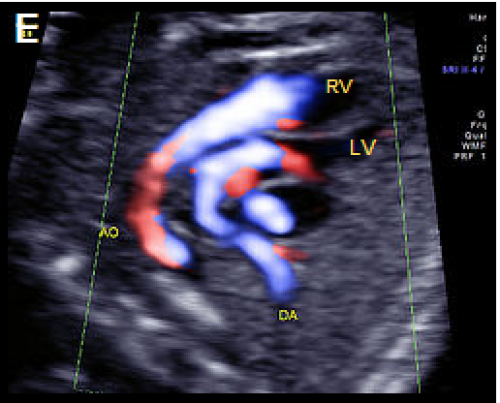

Oblique, apex-up view with colour Doppler showing the aorta arising from the (anterior) RV and the PA arising from the (posterior) LV.
2. Congenitally corrected transposition of the great arteries
Congenitally corrected TGA (cc-TGA) is a rare condition, with both AV and ventriculoarterial discordance (but normal veno-atrial connections).
- The morphologic right atrium connects to the morphologic left ventricle, and the morphologic left atrium connects to the morphologic right ventricle.
- The morphologic RV is characterised by the moderator band, more apical attachment of the AV valve, and shorter/triangular configuration. It is located left and posterior in cc-TGA and connects to the left atrium.
- The morphologic LV is located to the right and anterior in cc-TGA, has an elongated and smoother inner chamber and forms the apex of the heart.
- The great vessels are also discordant and transposed with a parallel course (the PA arises from the LV and the aorta from the RV).
- The aorta is anterior and to the left of the PA (hence also known as L-TGA).
- The associated AV and ventriculoarterial discordance results in haemodynamic compensation.
- This condition is more commonly associated with other cardiac anomalies than D-TGA (eg, VSD, pulmonary outflow obstruction, tricuspid valve anomalies, dextrocardia and cardiac arrhythmias).
- It is rarely associated with extra-cardiac anomalies or chromosomal anomaly.
- 22q11.2 microdeletion may be associated, particularly when cc-TGA is detected with other cardiac or extra-cardiac anomalies.
Common arterial trunk
Common arterial trunk (CAT) is also known as truncus arteriosus, persistent truncus arteriosus, truncus arteriosus communis and aorticopulmonary trunk. It is:
- characterised by a single ventriculoarterial trunk that gives rise to the pulmonary, systemic and coronary vessels
- almost always associated with a large VSD
- relatively uncommon, representing about 1 percent of congenital heart disease.
It may be classified into the following four types by the origin of the PAs, but this may be difficult prenatally.
- A short pulmonary trunk arises from the common arterial trunk and divides into right and left PAs.
- The PAs arise separately from the common arterial trunk, close to each other.
- The PAs arise separately from the common arterial trunk, distant from each other.
- The PAs arise from the aortic arch or descending aorta rather than from the common arterial trunk (recently reclassified as pulmonary atresia with VSD).
A dysplastic truncal valve is common.
Ultrasound findings
- The 4Ch view is often normal, unless there is a large VSD.
- CAT is best visualised on the LVOT view where a malaligned VSD and large overriding vessel is evident.
- A single large vessel is identified on scanning in the 3VT plane.
- The PA cannot be identified arising from the RV.
- The pulmonary trunk or branch PAs may be identified arising from the large overriding vessel.
- Colour Doppler may help in identifying the location of the PAs, demonstrating the VSD shunt and showing CAT valvular regurgitation.
Associations
The following cardiac anomalies are commonly associated.
- Perimembranous VSD is almost always identified
- The ductus arteriosus is absent in 50 percent of CAT cases
- Aortic arch abnormalities are common (right-sided aortic arch, interrupted arch and, less commonly, arch hypoplasia and double aortic arch)
- One PA is absent.
Extra-cardiac structural anomalies are present in about 40 percent of CAT cases.
Chromosomal anomalies are common, and include trisomies 21, 18 and 13 as well as microdeletion of 22q11.2 (in 30-40 percent of cases).
Image 26

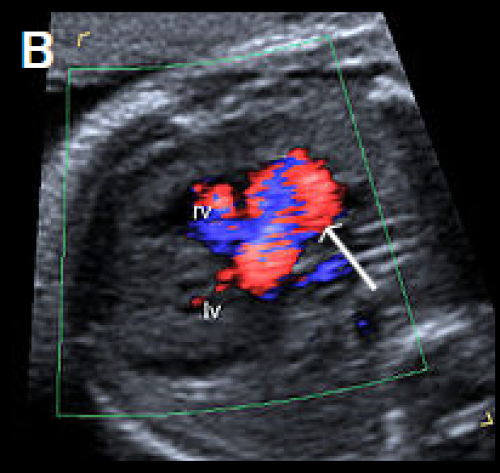

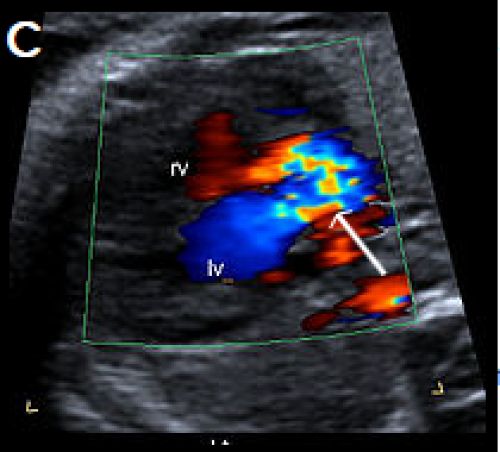

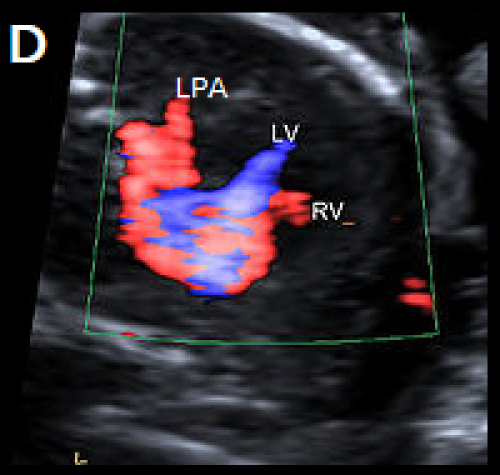


Single large central vessel (arrow) overriding both ventricles on greyscale (A) and colour Doppler imaging (B).
There is turbulent flow within the common arterial trunk on colour Doppler (C).
The left PA (D) and right PA, arrow (E), arise separately and distant from each other from the common arterial trunk (type 3 CAT).
Hypoplastic left heart syndrome and critical aortic stenosis
Hypoplastic left heart syndrome (HLHS) comprises a spectrum of congenital anomalies characterised by under-development or absence of the LV and LVOT.
There are varying degrees of hypoplasia of the LV, mitral valve and aortic valve atresia or stenosis, and hypoplasia of the ascending aorta.
Critical aortic stenosis may progress to HLHS.
Ultrasound findings in HLHS
B-mode
- The 4Ch view is abnormal with a variably small LV (absent, small or sometimes dilated), with reduced contractility and increased echogenicity of the inner wall due to endocardial fibroelastosis.
- The apex of the heart is predominantly formed by the RV.
- The aortic valve is atretic in most cases.
- The mitral valve is usually patent but may be dysplastic.
- The left atrium is small compared with the right atrium.
- The tricuspid valve may be dysplastic, and there may be tricuspid regurgitation.
- Foramen ovale leaflet motion may be paradoxical (from left to right).
- The LVOT is hypoplastic and may be difficult to visualise in the LVOT view.
- In the 3-vessel view, the pulmonary trunk appears dilated, and the aortic arch may be absent or hypoplastic.
Colour Doppler
- There is abnormal or absent filling of the hypoplastic LV and paradoxical left-to-right shunting across the foramen ovale.
- There is a lack of forward flow across the atretic aortic valve.
- There is an abnormal 3-vessel view, with antegrade flow within the dilated pulmonary trunk and reversed flow in the narrowed aortic arch.
- Retrograde flow is evident from the ductus arteriosus into the aortic isthmus.
Ultrasound findings in critical aortic stenosis
B-mode
- The LV is often abnormally dilated, with reduced function and increased echogenicity of the inner wall due to endocardial fibroelastosis.
- The dilated LV may still form the apex of the heart in this condition.
- The left atrium may also be dilated because of mitral valve regurgitation.
- The aortic root appears narrow on the LVOT view, and there may be reduced valvular motion.
Colour Doppler
- There may be evidence of mitral regurgitation.
- In severe cases, there may be reduced left ventricular filling and left-to-right shunting at the foramen ovale.
- Antegrade and often turbulent flow is seen across the severely stenotic but patent aortic valve, with peak velocities often >200 cm/s (however, reduced velocities and aortic regurgitation may also be evident and suggest left ventricular dysfunction).
- On the 3-vessel view, there may be antegrade or retrograde flow within the aortic isthmus, depending on severity.
Associations with HLHS
- Chromosomal anomalies can be associated with HLHS (including Turner syndrome and trisomies 13 and 18).
- Extra-cardiac abnormalities are overall less common in HLHS than in other congenital heart disease.
- Risk of recurrence is about 8 percent.
- Intrauterine growth restriction may be associated and is likely due to reduced cardiac output.
Associations with critical aortic stenosis
- Associated cardiac abnormalities are present in about 20 percent of cases and include tricuspid and aortic insufficiency, coarctation of the aorta and postnatal patent ductus arteriosus (PDA).
- Extra-cardiac and chromosomal abnormalities are rarely associated.
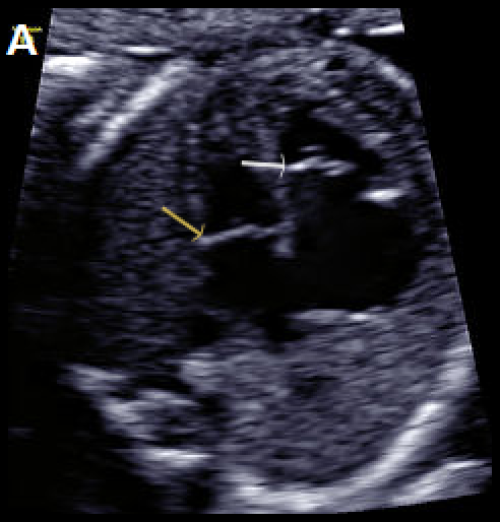
Image 27
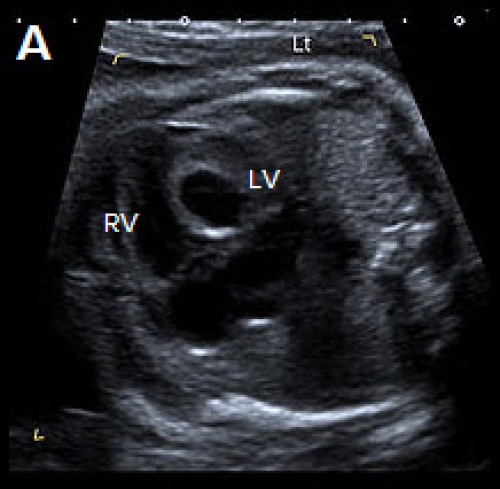




Abnormal small LV on the 4Ch view with an echogenic inner wall in keeping with endocardial fibroelastosis. The RV forms the apex of the heart (A).
Paradoxical left-to-right bulge of the foramen ovale flap, arrow (B). On colour Doppler imaging, there is minimal left ventricular filling (normal colour filling on the right, in red) (C), and mitral regurgitation, arrow (D).
Ebstein anomaly
- Ebstein anomaly is characterised by apical displacement of the septal and posterior tricuspid valve leaflets, which are attached to the walls and septum of the RV rather than to the (normally positioned) tricuspid valve annulus.
- This leads to ‘atrialisation’ of a portion of the morphologic RV, which is contiguous with the right atrium, causing the right atrium to be large and the anatomic RV to be small.
- The anomaly may be mild or severe.
- It is uncommon, accounting for 0.5–1 percent of congenital cardiac anomalies.
- Poor prognostic features include massive cardiomegaly, reduced right ventricular outflow from pulmonary stenosis, hydrops and detection before 20 weeks gestation.
Ultrasound findings
B-mode
- There is cardiomegaly, with right atrial enlargement. This may be subtle in the second trimester, but it may progress later in the pregnancy.
- The septal leaflet of the tricuspid valve may be identified arising more apically than usual from the ventricular wall rather than from the annulus.
- In severe forms, the IVS may demonstrate paradoxical movement, with the apical and basal septum moving in opposite directions.
- The pulmonary artery may be small with abnormal valvular excursion in cases with associated pulmonary stenosis or atresia.
Colour Doppler
- There is often tricuspid regurgitation (typically high velocity >200 cm/s and holosystolic).
- The regurgitant jet usually arises from the mid RV (in comparison to tricuspid dysplasia when the regurgitant jet arises at the level of the valve annulus).
- Assessment of the RVOT with colour Doppler may show reversed flow within the ductus arteriosus towards the pulmonary valve or antegrade flow into a narrow pulmonary artery, with associated pulmonary atresia or stenosis.
Associations
- Other cardiac anomalies are relatively common in association with Ebstein anomaly and include:
- pulmonary stenosis or atresia (possibly due to severe tricuspid regurgitation causing reduced flow across the pulmonary valve)
- atrial or ventricular septal defects.
- Supraventricular tachyarrhythmia may be associated but is usually a postnatal finding.
- Most cases are isolated but may be associated with chromosomal anomaly, for example, trisomy 21 or 13.
Image 28

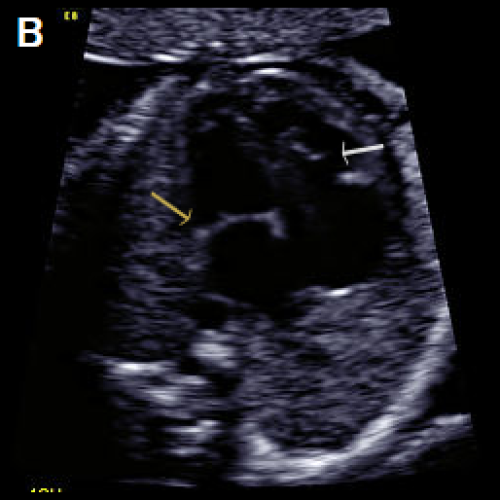

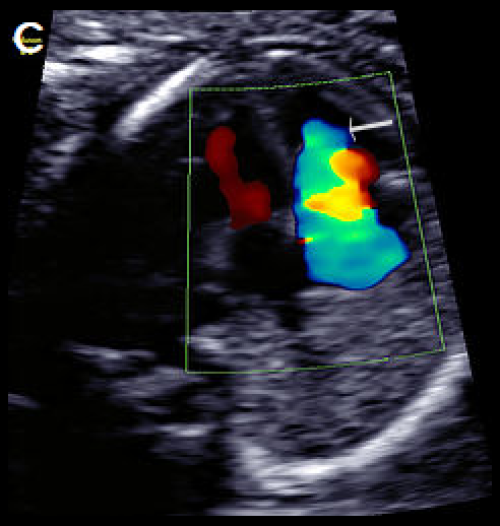

Greyscale 4Ch view in a fetus with Ebstein anomaly showing mild cardiomegaly and apical offset of the septal tricuspid valve leaflet (white arrow), with ‘atrialisation’ of the RV.
Normal mitral valve position (yellow arrow), in diastole (A), with the same fetus in systole, with the valve leaflets open (B).
On colour Doppler imaging, there is a large tricuspid regurgitant jet arising from the mid-apical RV, (arrow) (C).
Aortic arch obstruction
1. Coarctation of the aorta
- Coarctation is a relatively common anomaly occurring in 5–10 percent of neonates with congenital heart disease (CHD), characterised by narrowing of the aortic arch, usually at the isthmus, between the left subclavian artery and the ductus arteriosus.
- Tubular hypoplasia occurs when a long portion of the aortic arch is narrowed.
- Antenatal diagnosis may be difficult, particularly in the second trimester. The condition may not present until after closure of the ductus in neonatal life.
- Prenatal ultrasound has a poor positive and negative predictive value in detecting the lesion. This may be improved by measuring the isthmus and plotting it on a normogram (see Matsui et al 2008), calculating the isthmic: arterial duct ratio and assessing the isthmus with pulsed Doppler.
Ultrasound findings
B-mode
- Ventricular disproportion, with a narrower LV compared with the right, may be evident on the 4Ch view.
- Left ventricular contractility and the mitral valve are normal (unlike HLHS).
- The LVOT view is typically normal with a normal calibre ascending aorta.
- On the 3-vessel view, the transverse aortic arch is narrow compared with the PA. A persistent left SVC, if present, may also be identified in this plane.
- The extent and location of narrowing is best appreciated in the longitudinal aortic arch view (and most commonly involves the arch between the left subclavian artery and the origin of the ductus arteriosus).
- With severe coarctation, the transverse arch between the left common and left subclavian arteries may be narrowed and elongated, and the left subclavian artery arises at the junction of the ductus arteriosus with the descending aorta.
Colour Doppler
- There is normal filling of the LV in diastole on the 4Ch view (in contrast to HLHS).
- It shows forward flow across the aortic valve in the LVOT view.
- It demonstrates the isthmic narrowing on the longitudinal view, as well as the typical ‘shelf’ appearance at the junction of the ductus and descending aorta. This may be best appreciated with power Doppler.
- There is a narrow transverse arch on the 3-vessel view, with more pronounced narrowing towards the isthmus.
- Despite the narrowing, velocities are usually not increased, and colour aliasing is not usually present.
Associations
- Other cardiac anomalies, particularly large VSD. Other associated anomalies include aortic stenosis, bicuspid aortic valve, mitral stenosis.
- Persistent left SVC may be associated with coarctation, and this finding should prompt follow-up cardiac examination when there is subtle ventricular discrepancy.
- There can be chromosomal anomaly especially Turner syndrome and trisomies 13 and 18.
- Extra-cardiac abnormalities are common and include vascular anomalies and berry aneurysms.
Beware: Severe fetal growth restriction may be associated with a narrowed isthmus due to shunting of blood and may be misinterpreted as coarctation.
Image 29
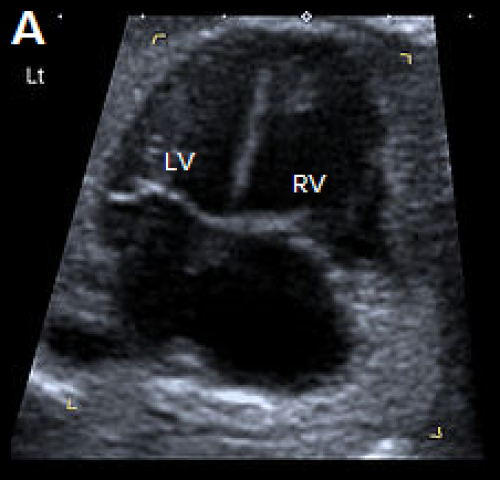

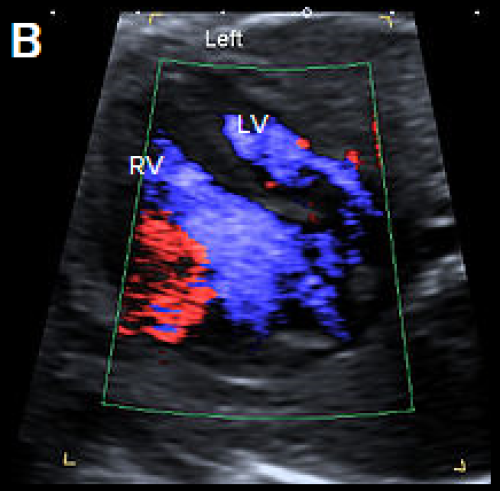

4Ch heart view showing narrower LV compared with the RV, on greyscale imaging (A), and with a narrower colour strip and patent AV valves on colour Doppler imaging (B).
Image 30
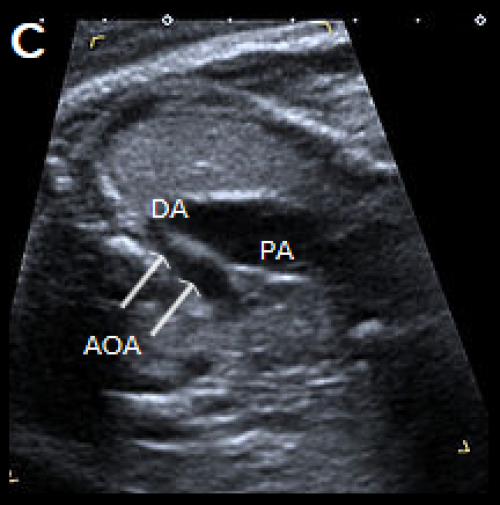

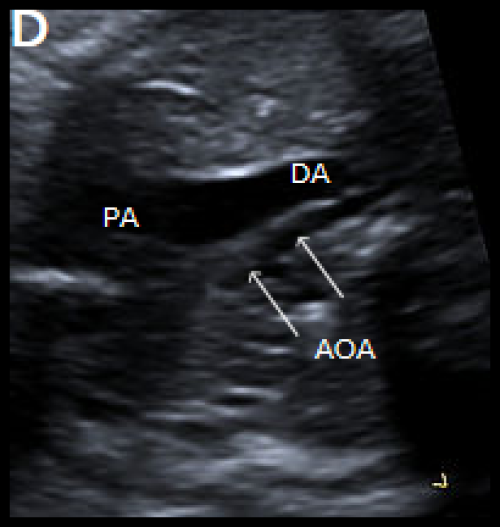

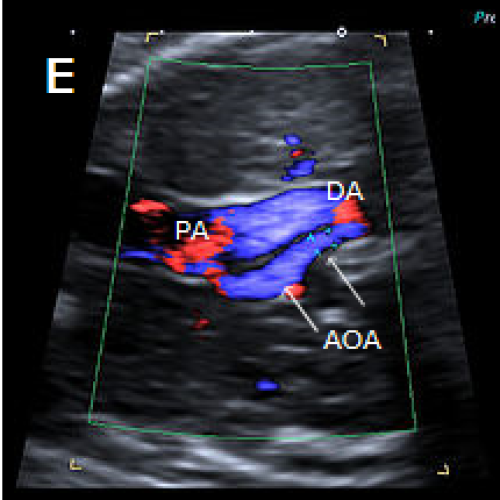

3-vessel view in two different fetuses with coarctation (C) and tubular hypoplasia of the aortic arch (D), with a narrow aortic arch compared with the PA.
Colour Doppler confirms the narrow calibre of the aortic arch compared with the PA (E).
Image 31
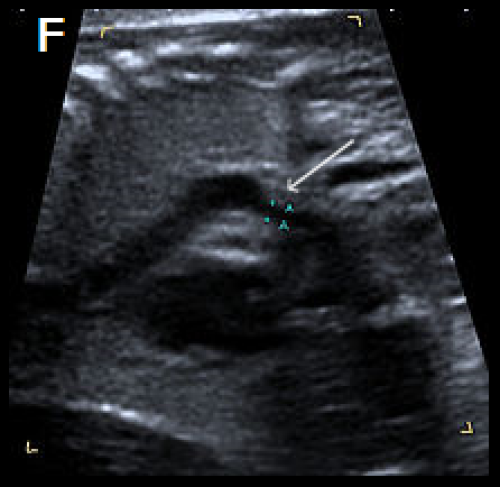

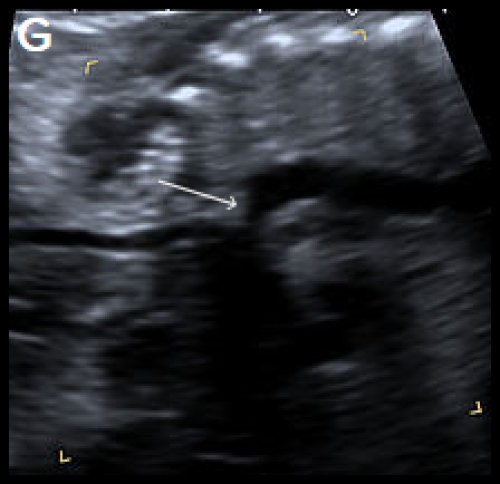

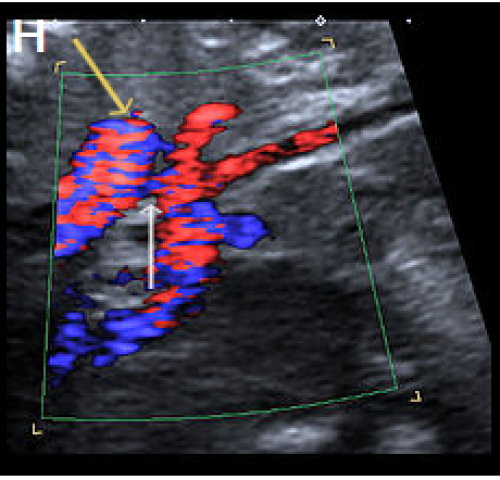

Sagittal views of the aortic arch in greyscale in two different fetuses, showing narrowing in the isthmic region (arrow) (F) and (G) and in colour Doppler (white arrow) (H), with the coarctation ‘shelf’ at the junction of the ductus and descending aorta (yellow arrow).
2. Interruption of the aortic arch
Interruption of the aortic arch (IAA) is a rare cardiac anomaly (comprising about 1 percent of CHD) in which there is incomplete development of the aorta, with a gap between the ascending and descending thoracic aorta.
It is almost always found in association with other cardiac anomalies, including VSD, aorto-pulmonary window and CAT at the junction of the ductus and descending aorta.
IAA may be classified by the anatomic location of the site of interruption as follows.
- Type A: The aortic arch is interrupted after the left subclavian artery.
- Type B: The aortic arch is interrupted between the left common carotid artery and the left subclavian artery. This is the most common form of IAA and the type most frequently associated with 22q11.2 deletion.
- Type C: The aortic arch is interrupted between the brachiocephalic artery and the left common carotid artery. This is the least common type of IAA.
Ultrasound findings
B-mode
- Unlike coarctation, the left ventricular size is usually normal on the 4Ch view (particularly when associated with a large VSD).
- On the LVOT view, a small aortic root VSD may be evident.
- The 3-vessel view will be abnormal, with loss of continuity of the transverse aortic arch. The pulmonary trunk may appear slightly dilated.
- On the 3-vessel view, the thymus may be hypoplastic or absent (the PA abuts the sternum without normal intervening thymic tissue).
- The longitudinal view fails to show the continuous ‘candy-cane’ appearance of the aortic arch. The aorta may have a straight course, continuing into the brachiocephalic and left common carotid arteries.
Colour Doppler
- The 4Ch and LVOT views may confirm a VSD.
- It will demonstrate normal flow across the aortic valve.
- There will be loss of continuity and straight course of the aorta towards the head and neck vessels on longitudinal views.
- The left subclavian artery will be arising from the ductus arteriosus (in type B).
- There may be an aberrant right subclavian artery coursing posterior to the trachea.
Associations
- Other cardiac anomalies, particularly VSD (in 90 percent of cases), right-sided aortic arch and aberrant subclavian arteries. Other associated cardiac anomalies include aorto-pulmonary window, AVSD, single ventricle and double outlet right ventricle.
- There could be 22q11.2 deletion (especially type B, in about 50 percent of cases).
Image 32
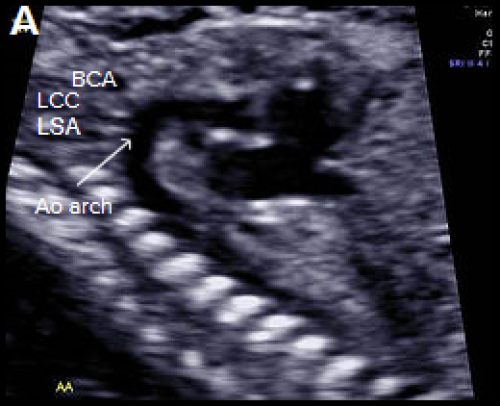

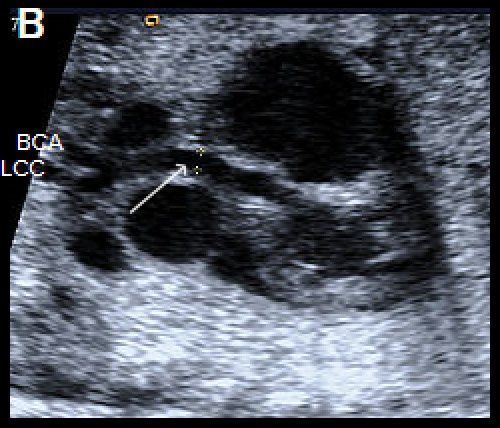



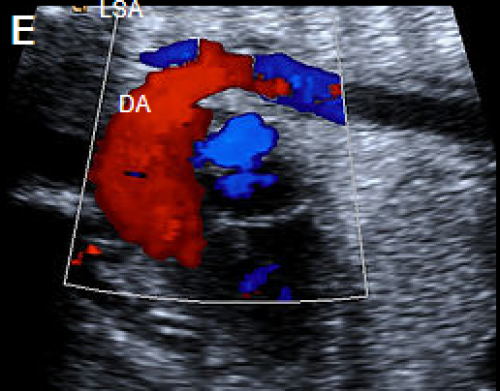




Longitudinal view of a normal aortic arch (A) showing the normal branching of the head and neck vessels. Brachiocephalic artery (BCA), left common carotid artery (LCC), left subclavian artery (LSA).
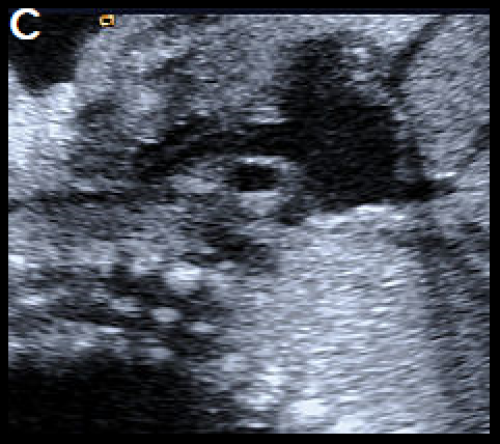
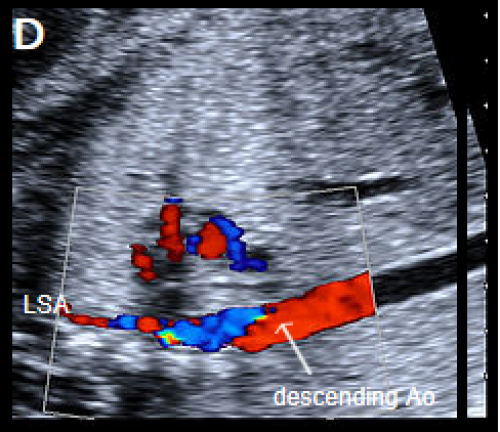
A fetus with IAA showing an elongated straight course of the small calibre aorta (arrow), continuing into the brachiocephalic and LCC arteries (B), and loss of continuity of the transverse arch in greyscale (C), and colour Doppler (D). The LSA arises from the ductus arteriosus (DA), (E).
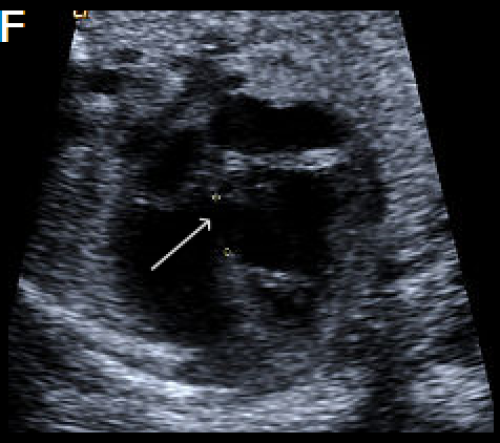
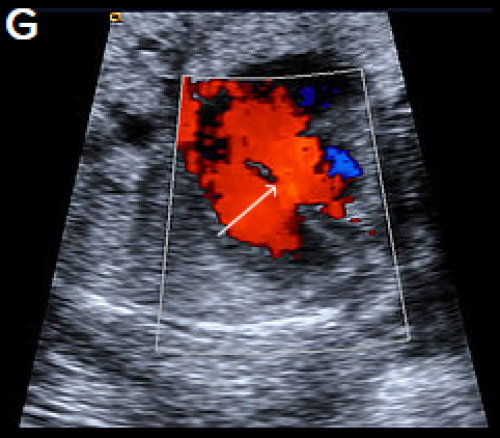
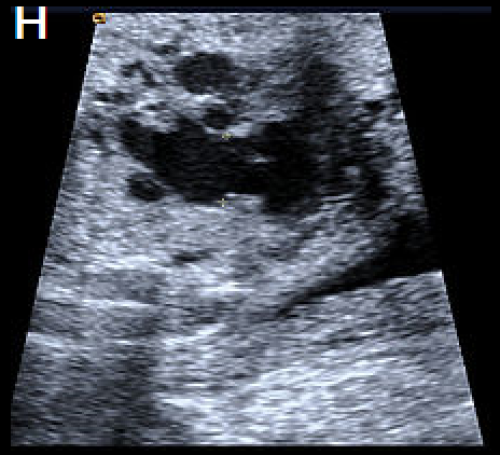
A perimembranous VSD is evident on greyscale (F), and colour Doppler (G), arrowed. Note that the ventricles are congruent in size. The RVOT appears dilated compared with the small aorta (H).