On this page
Key information
|
Mode of transmission |
Contact with respiratory droplets or infected skin of a case or carrier or, more rarely, contaminated objects. |
|
|---|---|---|
|
Incubation period |
Usually 2–5 days, occasionally longer. |
|
|
Period of communicability |
Variable; usually 2 weeks or less, seldom more than 4 weeks. Carriers may shed for longer. Effective antimicrobial therapy promptly terminates shedding. |
|
|
Funded and available vaccines |
DTaP-IPV-HepB/Hib (Infanrix-hexa). DTaP-IPV (Infanrix-IPV). Tdap (Boostrix). |
|
|
Dose, presentation, route |
0.5 mL per dose. DTaP-IPV-HepB/Hib: pre-filled syringe and glass vial. The vaccine must be reconstituted prior to injection. DTaP-IPV, Tdap: pre-filled syringe. Intramuscular injection. |
|
|
Funded vaccine indications and schedule |
During each pregnancy (recommended from 16 weeks’ gestation) for pertussis protection |
Tdap |
|
6 weeks, 3 months and 5 months |
DTaP-IPV-HepB/Hib |
|
|
4 years |
DTaP-IPV |
|
|
11 years |
Tdap |
|
|
45 years (catch-up, if individual has not received 4 previous tetanus doses) |
Tdap |
|
|
65 years |
Tdap |
|
|
Parents or primary caregivers of infants admitted to neonatal intensive or specialist baby care units for more than 3 days and whose mothers had not received Tdap at least 14 days prior to birth for pertussis protection |
Tdap |
|
|
For vaccination of previously unimmunised or partially immunised patients |
DTaP‑IPV‑HepB/Hib, DTaP-IPV or Tdap |
|
|
For (re)vaccination of eligible patients |
||
|
Vaccine effectiveness |
Protection of 87–98 percent has been demonstrated using population-based analysis. Immunised cases have been shown to have less severe disease To prevent major community outbreaks at least 70 percent of the childhood population must be immune to diphtheria |
|
|
Public health measures |
All cases of diphtheria must be notified immediately on suspicion (see section 6.8). |
|
|
Post-exposure prophylaxis |
Antimicrobial prophylaxis should be administered to contacts as appropriate (see section 6.8). |
|
6.1. Bacteriology
Diphtheria is a serious, often fatal, toxin-mediated disease caused by Corynebacterium diphtheriae, a non-sporulating, non-encapsulated, gram-positive bacillus. Rarely, it may also be caused by other toxin-carrying Corynebacteria species, such as Corynebacterium ulcerans. Both toxigenic and non-toxigenic strains can occur during outbreaks, during which non-toxigenic strains can convert to toxigenic strains by infection with tox-gene containing β-corynebacteriophage.[1]
6.2. Clinical features
Classic diphtheria characteristically involves membranous inflammation of the upper respiratory tract with involvement of other tissues, especially the myocardium and peripheral nerves. The organism itself is rarely invasive, but a potent exotoxin, diphtheria toxin, is produced by some toxigenic strains that causes tissue damage through local and systemic actions. There is also a cutaneous form of diphtheria, which is typically less severe. The detection of either C. diphtheriae or C. ulcerans is notifiable to the medical officer of health, and the isolates should be referred to the Institute of Environmental Science and Research (PHF Science (formerly ESR)) for toxin detection. Non-toxigenic diphtheria strains can circulate but these do not secrete diphtheria toxin and are not associated with disease nor preventable by immunisation with diphtheria toxoid vaccine.
Transmission is by respiratory tract droplets, or by direct contact with skin lesions or contaminated articles. Cutaneous toxigenic diphtheria is more efficiently transmitted than respiratory toxigenic diphtheria and can cause respiratory infection in others.[2, 3] Humans are the only known host for diphtheria, and the disease is usually spread by close personal contact with a case or carrier, or occasionally by fomites or food. The disease remains communicable for up to four weeks after infection, but carriers of C. diphtheriae may continue to shed the organism and be a source of infection for much longer.
Classic diphtheria has a gradual onset after an incubation period of two to five days. Symptoms and signs may be mild at first, but progress over one to two days with the development of a mildly painful tonsillitis or pharyngitis with an associated greyish membrane. Diphtheria should be suspected particularly if the membrane extends to the uvula and soft palate. The nasopharynx may also be obstructed by a greyish membrane, which leaves a bleeding area if disturbed. The breath of a patient with diphtheria has a characteristic mousy smell.
The major complication of diphtheria is respiratory obstruction, although most deaths result from the effects of diphtheria toxin on various organs. Of importance is the toxicity to the myocardium (leading to myocarditis and heart failure), peripheral nerves (resulting in demyelination and paralysis), and kidneys (resulting in tubular necrosis). The neuropathy begins two to eight weeks after disease onset, while the myocarditis can be early or late. Neurological complications occur in 15–20 percent of cases and can cause paralysis up to two months after disease onset.[1]
6.3. Epidemiology
6.3.1. Global burden of disease
6.3.1. Global burden of disease
In the pre-immunisation era, diphtheria was predominantly a disease of children aged under 15 years; most adults acquired immunity without experiencing clinical diphtheria. Asymptomatic carriage was common (3–5 percent) and important in perpetuating both endemic and epidemic diphtheria. The global incidence of diphtheria dropped dramatically during the 20th century. Immunisation played a large part but may not be wholly responsible for this reduction (see Figure 6.1). The estimated total number of diphtheria cases globally fell from just under 100,000 cases in 1980 to around 4,500 in 2015, but increased to over 16,500 cases in 2018.[4]
Diphtheria remains a significant health issue in countries with low immunisation coverage. Around 14 percent of children globally are not fully immunised against diphtheria and all countries have pockets of unvaccinated children.[5] Most cases of diphtheria reported during 2011–2015 were in South-East Asia, in particular India and Indonesia, but under-reporting has been shown for Africa and Eastern Mediterranean regions.[5]
Figure 6.1: Diphtheria global annual reported cases and DTP3* immunisation coverage, 1980–2018
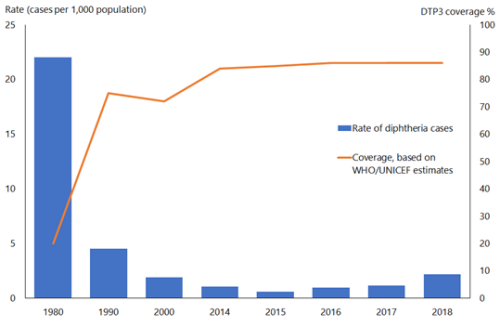
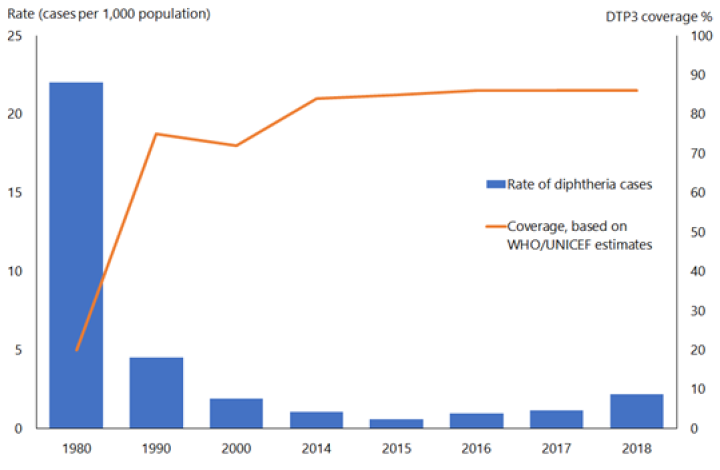
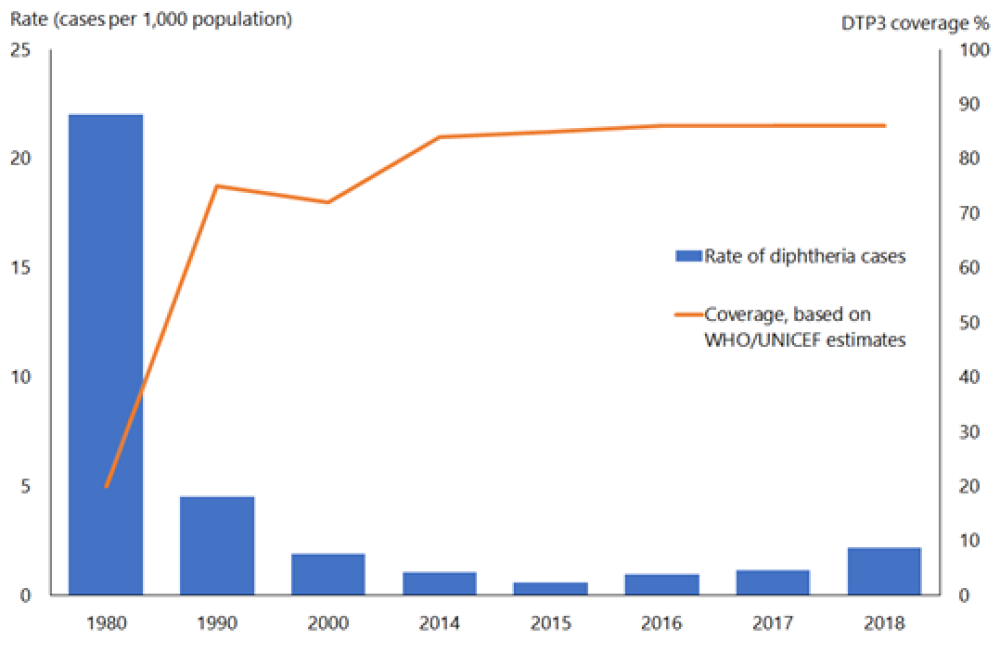
*Three doses of diphtheria, tetanus and pertussis vaccine in infancy
Source: WHO/UNICEF as of December 2019
Immunisation leads to the disappearance of circulating toxigenic strains, but non-toxigenic strains infected with the β-corynebacteriophage can become toxigenic during outbreaks. The return of epidemic diphtheria is a real threat when herd immunity is insufficient, as happened in the states of the former Soviet Union during 1990–1997. Factors contributing to this epidemic included a large population of susceptible adults, decreased childhood immunisation, suboptimal socioeconomic conditions and high population movement.[6]
Most reported cases were unvaccinated or incompletely vaccinated adolescents and adults (40 percent of cases were age over 15 years in high-incidence countries and 66 percent in low incidence countries) due to historically low coverage and a lack of booster doses to maintain protection in adolescents and adults. This vulnerability in adults was highlighted by a recent outbreak in Indonesia.[7] However, continuing endemic cutaneous diphtheria in indigenous communities has been reported from the US, Canada and Australia. Small diphtheria outbreaks occurring in high-income countries often appear to be caused by non-immune individuals travelling to endemic countries.[1]
The overall case fatality rate for clinical diphtheria is 5–10 percent, with higher death rates (up to 20 percent) among persons younger than 5 and older than 40 years. The case-fatality rate for diphtheria has changed very little during the last 50 years.[8]
Diphtheria remains rare in high-income countries such as New Zealand due to widespread use of diphtheria toxoid-containing vaccines. Falling immunisation coverage rates worldwide increases the risk for diphtheria outbreaks.
6.3.2. New Zealand epidemiology
6.3.2. New Zealand epidemiology
Diphtheria infection was common in New Zealand until the 1960s. The last case of toxigenic respiratory diphtheria was reported in 1998.[9] Low numbers of cutaneous toxigenic diphtheria are regularly notified in New Zealand: two confirmed cases were notified in 2015 in refugees from Afghanistan[10] and one case was notified in 2019 in an immunised child from Papua New Guinea.
Non-toxigenic C. diphtheria isolates continue to be identified; for example, there were seven throat and 41 cutaneous non-toxigenic isolates and seven from other sites in 2019 (ESR, 8 June 2020). These strains do not cause diphtheria disease.
Travel to endemic countries is an important risk factor for infection, but transmission within New Zealand can occur to susceptible contacts of cutaneous cases. Tattooing practices in the Pacific Islands have also been implicated in outbreaks in New Zealand.[11]
The 2005–2007 National Serosurvey of Vaccine Preventable Diseases found that 61 percent of 6–10-year-olds, 77 percent of 11–15-year-olds, 71 percent of 16–24-year-olds, 48 percent of 25–44-year-olds and 46 percent of ≥45-year-olds had presumed protective levels of diphtheria antibody.[12] The decline apparent with age suggests there is likely to be a large and increasing pool of older adults who may be susceptible to diphtheria in New Zealand, despite the introduction of adult tetanus and diphtheria (Td) vaccination in 1994.
For details of diphtheria notifications, refer to the most recent PHF Science (formerly ESR) notifiable disease annual tables and reports. (external link)
6.4. Vaccines
Diphtheria toxoid is prepared from cell-free purified diphtheria toxin treated with formaldehyde. It is a relatively poor immunogen, which, to improve its efficacy, is usually adsorbed onto an adjuvant, either aluminium phosphate or aluminium hydroxide.
Diphtheria toxoid is only available as a component of combination vaccines (in New Zealand as DTaP-IPV-HepB/Hib, DTaP-IPV and Tdap).
See Appendix 1 for the history of diphtheria toxoid-containing vaccines in New Zealand.
6.4.1. Available vaccines
6.4.1. Available vaccines
Funded diphtheria vaccines
The diphtheria toxoid-containing vaccines funded as part of the Schedule are as follows.
DTaP-IPV-HepB/Hib (Infanrix-hexa, GSK): diphtheria, tetanus, acellular pertussis, inactivated polio, hepatitis B and Haemophilus influenzae type b vaccine, which contains:
- not less than 30 IU of diphtheria and 40 IU of tetanus toxoids and three purified Bordetella pertussis antigens (25 µg of pertussis toxoid; 25 µg of filamentous hemagglutinin; 8 µg of pertactin, a 69 kilodalton outer membrane protein) adsorbed onto aluminium salts
- three types of inactivated polio viruses: 40 D-antigen units of type 1 (Mahoney), 8 D antigen units of type 2 (MEF-1) and 32 D‑antigen units of type 3 (Saukett)
- 10 µg of purified major surface antigen (HBsAg) of the hepatitis B virus (HBV)
- 10 µg of purified polyribosylribitol phosphate (PRP) capsular polysaccharide of Haemophilus influenzae type b (Hib), covalently bound to 20–40 µg tetanus toxoid, adsorbed onto aluminium salts
- lactose, sodium chloride, Medium 199, potassium chloride, disodium phosphate, monopotassium phosphate, polysorbate 20 and 80, glycine, formaldehyde, neomycin sulphate and polymyxin B sulphate, which are also present as other components or as trace residuals from the manufacturing process.
DTaP-IPV (Infanrix-IPV, GSK): diphtheria, tetanus, acellular pertussis and inactivated polio vaccine, in the same quantities as for Infanrix-hexa above. Other components and residuals include sodium chloride, aluminium salts, Medium 199, potassium chloride, disodium phosphate, monopotassium phosphate, polysorbate 80, glycine, formaldehyde, neomycin sulphate and polymyxin B sulphate.
Tdap (Boostrix, GSK): a smaller adult dose of diphtheria toxoid and pertussis antigens together with tetanus toxoid. Tdap contains not less than 2 IU of diphtheria toxoid, not less than 20 IU of tetanus toxoid, 8 µg of pertussis toxoid, 8 µg of filamentous hemagglutinin and 2.5 µg of pertactin, adsorbed onto aluminium salts. Other components and trace residuals include sodium chloride, formaldehyde, polysorbate 80 and glycine.
Other vaccines
Other diphtheria toxoid-containing vaccines registered (approved for use) and available (marketed) in New Zealand are:
- Tdap: Adacel (Sanofi)
- Tdap-IPV: Adacel Polio (Sanofi).
6.4.2. Efficacy and effectiveness
6.4.2. Efficacy and effectiveness
Immunity against toxigenic diphtheria occurs via an antibody‐mediated response to the diphtheria toxin and is primarily of the IgG type. Antitoxin antibodies can pass through the placenta to provide passive immunity to the newborn.
Although there are no randomised controlled studies on the efficacy of the vaccine, between 87 and 98 percent protection has been demonstrated using population-based analyses. Immunised cases have been shown to have less severe disease, as highlighted during the outbreak in the former Soviet Union.
Vaccines combining pertussis antigens with diphtheria and tetanus toxoids have been gradually introduced into immunisation schedules throughout the world. Immunogenicity data for these combination vaccines is discussed in section 16.4.2.
Herd immunity
Immunisation is more effective at preventing disease, by neutralising diphtheria toxin, than preventing infection. Herd immunity is created by reducing carriage and transmission from symptomatic patients.[13, 14] To prevent major community outbreaks, it has been suggested that 70 percent or more of the childhood population must be immune to diphtheria.[15, 16] Herd immunity in adults is likely to depend on the size of the reservoir of disease in children.[1] This may explain the control of diphtheria in New Zealand despite historically relatively poor coverage.
Duration of immunity
Diphtheria antitoxin levels decline over time in children after they have received a primary series of vaccines, and a booster dose is required. In countries where diphtheria immunisation is common practice and high coverage rates are achieved, there will be no natural boosting from circulating disease, and antitoxin levels declining with increasing age may result in a susceptible older adult population.[5] WHO identified that adults in countries with long-standing childhood immunisation programmes were likely to be susceptible without booster doses to overcome waning immunity due to lack of exposure to diphtheria. Waning immunity contributed to the high number of cases in adults observed during the outbreak in the former Soviet Union.[17]
Despite apparent immunity gaps, there has been minimal disease in high-income countries, suggesting that the established correlates of seroprotection based on antitoxin levels may not be reliable guides for protection and that other factors may be operating.[18] For example, a high proportion of the adult German population have low antibody levels, indicating susceptibility, yet diphtheria outbreaks have not occurred despite Germany’s relative geographical proximity to high rates of disease in the former Soviet Union.[19]
The duration of protection after Tdap boosters is unknown, but the results of an Australian study have shown that five years after the Tdap booster dose, 94.4 percent of adults had seroprotective levels of antibodies against diphtheria, compared with 93.7 percent who received Td vaccine.[20] It has been predicted that 95 percent of the adult population in the US would maintain seroprotection for more than 30 years without requiring further booster doses.[21]
6.4.3. Transport, storage and handling
6.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
Store at +2°C to +8°C. Do not freeze. DTaP-IPV-HepB/Hib and Tdap should be stored in the dark.
DTaP-IPV-HepB/Hib (Infanrix-hexa) must be reconstituted by adding the entire contents of the supplied container of the DTaP‑IPV-HepB vaccine to the vial containing the Hib-PRP pellet. After adding the vaccine to the pellet, the mixture should be shaken until the pellet is completely dissolved. Use the reconstituted vaccine as soon as possible. If storage is necessary, the reconstituted vaccine may be kept for up to eight hours at 21°C.
6.4.4. Dosage and administration
6.4.4. Dosage and administration
The dose of DTaP-IPV-HepB/Hib, DTaP-IPV or Tdap vaccine is 0.5 mL, administered by intramuscular injection (see section 2.2.3).
Co-administration with other vaccines
DTaP-IPV-HepB/Hib, DTaP-IPV or Tdap vaccine can be administered simultaneously (at separate sites) with other vaccines or IGs.
6.5. Recommended immunisation schedule
Table 6.1: Immunisation schedule for diphtheria-containing vaccines (excluding catch-up)
Table 6.1: Immunisation schedule for diphtheria-containing vaccines (excluding catch-up)
|
Age |
Vaccine |
Comment |
|---|---|---|
|
Pregnant women – recommended from 16 weeks’ gestation of every pregnancy, preferably in the second trimester (funded when given any time in second or third trimester) |
Tdap |
Booster for mothera |
|
6 weeks |
DTaP-IPV-HepB/Hib |
Primary series |
|
3 months |
DTaP-IPV-HepB/Hib |
Primary series |
|
5 months |
DTaP-IPV-HepB/Hib |
Primary series |
|
4 years |
DTaP-IPV |
Booster |
|
11 years |
Tdap |
Booster |
|
45 years (individuals who have not received 4 tetanus vaccinations in their lifetime) |
Tdap |
Booster |
|
65 years |
Tdap |
Booster |
|
a. Tdap booster during pregnancy is to protect against mother and newborn against pertussis (see section 4.1.2). |
||
6.5.1. Usual childhood schedule
6.5.1. Usual childhood schedule
A primary course of diphtheria vaccine is given as DTaP-IPV-HepB/Hib (Infanrix-hexa) at ages 6 weeks, 3 months and 5 months, followed by a dose of DTaP-IPV (Infanrix-IPV) at age 4 years (Table 6.1). A booster is given at age 11 years (school year 7), which includes a pertussis component given as the vaccine Tdap (Boostrix).
If a course of immunisation is late or interrupted for any reason, it may be resumed without repeating prior doses (see Appendix 2).
6.5.2. Catch-ups for individuals aged 10 years and older
6.5.2. Catch-ups for individuals aged 10 years and older
For previously unimmunised individuals aged 10 years and older, a primary immunisation course consists of three doses of a diphtheria toxoid-containing vaccine at intervals of not less than four weeks (see Appendix 2). For children aged under 18 years, a booster dose is recommended at least six months after the third dose.
Children aged under 18 years may receive Tdap (funded from age 7 to under 18 years); adults aged 18 years and older may receive Tdap (funded). Although Tdap is not approved for use (registered) as a primary course, there are expected to be no safety concerns.
6.5.3. Booster doses for adolescents and adults
6.5.3. Booster doses for adolescents and adults
Studies overseas show that many adults lack protective levels of the antitoxin, and this has led to concern about waning immunity and recommendations for booster doses beyond childhood (see also section 6.3.2). Most authorities recommend maintaining diphtheria immunity by periodic reinforcement using a combined Td vaccine.[1] A single booster dose of Tdap induces seroprotective levels of antibodies to diphtheria and tetanus in virtually all children and adolescents, and in a high proportion of adults and elderly individuals at approximately one month post‐vaccination, irrespective of their vaccination history.[22]
Tdap is recommended and funded:
- as a booster dose to all adolescents at school year 7 or age 11 years
- as a booster dose for vaccination of individuals aged 65 years old
- as a single dose for catch-up vaccination of individuals aged 45 years old who have not had four previous tetanus-containing doses.
- for vaccination of previously unimmunised or partially immunised patients
- for vaccination prior to planned or revaccination following immunosuppression (see section 6.5.5)
- for boosting of patients with tetanus-prone wounds (see section 22.5.5).
These age-specific recommendations may facilitate the linkage of adult immunisation to the delivery of other preventive health measures.
Booster doses before travel
If someone is travelling to an area endemic for diphtheria, or there is another reason to ensure immunity, a booster dose is recommended (but not funded) if it is more than 10 years since the last dose. For website sources on travel vaccines, see Appendix 8.
6.5.4. Pregnancy and breastfeeding
6.5.4. Pregnancy and breastfeeding
Pregnant women should receive a dose of Tdap in every pregnancy so that antibodies can pass to the fetus to provide pertussis protection from birth (funded when given any time in second or third trimester). It is recommended to be given from 16 weeks’ gestation of every pregnancy, preferably in the second trimester to protect both the mother and her infant from pertussis (see section 16.5.2).[23]
Tdap vaccine may also be given to pregnant women when catch-up is needed for an under-immunised woman, or for management of a tetanus-prone wound (see section 22.5.5).[23, 24]
Tdap vaccines can be given to breastfeeding women.[24]
6.5.5. (Re)vaccination
6.5.5. (Re)vaccination
Diphtheria toxoid-containing vaccines are funded for (re)vaccination of eligible patients as follows, including prior to planned or following immunosuppression. See also sections 4.3.6 and 4.3.7.
DTaP-IPV-HepB/Hib (Infanrix-hexa) and DTaP-IPV (Infanrix-IPV)
Up to an additional four doses (as appropriate) of DTaP-IPV-HepB/Hib (for children aged under 10 years) or DTaP-IPV are funded for (re)vaccination of patients:
- post-HSCT or chemotherapy
- pre- or post-splenectomy
- pre- or post-solid organ transplant
- undergoing renal dialysis
- prior to planned or following other severely immunosuppressive regimens.
Up to an additional four doses (as appropriate) of DTaP-IPV-HepB/Hib are funded for children aged 10 years to under 18 years for (re)vaccination of patients post HSCT. This is an off-label use of this vaccine which requires a prescription from an authorised prescriber or the patient’s specialist. In this group, all prior immunity will have been lost and revaccination is equivalent to a primary immunisation schedule. DTaP-IPV-HepB/Hib not recommended for other severely immunocompromised individuals aged 10-18 years that require revaccination as they may have residual immune memory.
Tdap (Boostrix)
An additional four doses (as appropriate) of Tdap (Boostrix) are funded for patients:
- post-HSCT or chemotherapy
- pre- or post-splenectomy
- pre- or post-solid organ transplant
- undergoing renal dialysis
- prior to planned or following other severely immunosuppressive regimens.
6.6. Contraindications and precautions
See also section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 for general contraindications for all vaccines.
6.6.1. Contraindications
6.6.1. Contraindications
There are no specific contraindications to diphtheria vaccine (as Tdap or DTaP), except for anaphylaxis to a previous dose or any component of the vaccine.
Most other cases of hypersensitivity that have been reported for tetanus-containing vaccines were in individuals who have had an excessive number of booster injections outside the guidelines noted above (see section 22.6.2).
6.6.2. Precautions
6.6.2. Precautions
See section 16.6.2 for precautions for pertussis-containing vaccines and section 22.6.2 for precautions for tetanus-containing vaccines.
6.7. Potential responses and AEFIs
Despite the widespread use of diphtheria toxoid, the 1994 Institute of Medicine review of vaccine reactions did not identify any reaction for which the evidence favoured or established a causal relationship with diphtheria toxoid.[25] However, local and systemic reactions do occur with diphtheria toxoid-containing vaccine, especially when the infant vaccine is used in older children and adults. Mild discomfort or pain at the injection site persisting for up to a few days is common.[24]
See also sections 16.7 and 22.7 for potential responses and AEFIs to DTaP‑IPV‑HepB/Hib, DTaP-IPV and Tdap.
6.8. Public health measures
It is a legal requirement that all cases of diphtheria be notified immediately on suspicion to the local medical officer of health. Notification should not await confirmation.
All isolates of C. diphtheriae and C. ulcerans are notifiable until toxigenicity is determined, including cutaneous isolates. If the isolate is determined to be non-toxigenic, the case should be de-notified.
All contacts should have cultures taken. Contacts who have a positive laboratory result should be isolated as if they are a case until proven bacteriologically negative.
For detailed information about public health control measures and case definition see the diphtheria chapter of the Communicable Disease Control Manual.
6.8.1. Antimicrobial prophylaxis
6.8.1. Antimicrobial prophylaxis
All contacts, after cultures have been taken and regardless of immunisation status, require antimicrobial prophylaxis.
For more information see the Communicable Disease Control Manual.
6.8.2. Vaccination of contacts
6.8.2. Vaccination of contacts
All close contacts should also be offered a complete course of vaccine or a booster according to the following schedule.
- Fully immunised children up to and including 6 years of age who have only received three doses of diphtheria toxoid-containing vaccine: give one injection of a DTap-IPV.
- Fully immunised individuals aged 7 years and older who have not received a booster dose of a diphtheria toxoid-containing vaccine within the last five years: give one injection of Tdap – funded if aged 10–17 years; unfunded if aged 18 years or older (see section 6.5).
- Unimmunised individuals: see Appendix 2.
6.9. Variations from the vaccine data sheets
See section 16.9 for variations from the DTaP-IPV-HepB/Hib (Infanrix-hexa), DTaP-IPV (Infanrix-IPV) and Tdap (Boostrix) data sheets.
References
References
References
- Tiwari TSP ,Wharton M. 2018. Diphtheria Toxoid, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- Belsey MA, Sinclair M, Roder MR, et al. Corynebacterium diphtheriae skin infections in Alabama and Louisiana. A factor in the epidemiology of diphtheria. New England Journal of Medicine, 1969. 280(3): p. 135-41.
- Koopman JS ,Campbell J. The role of cutaneous diphtheria infections in a diphtheria epidemic. Journal of Infectious Diseases, 1975. 131(3): p. 239-44.
- World Health Organization. Diphtheria reported cases to 2018. [updated 10 December 2019 ]; URL: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencediphtheria.html (external link). (accessed 19 February 2020 )
- World Health Organization. Diphtheria vaccine: WHO position paper - August 2017. Weekly Epidemiological Record, 2017. 92(31): p. 417-35.
- Vitek CR ,Wharton M. Diphtheria in the former Soviet Union: reemergence of a pandemic disease. Emerging Infectious Diseases, 1998. 4(4): p. 539-50.
- Rengganis I. Adult Diphtheria Vaccination. Acta Medica Indonesiana, 2018. 50(3): p. 268-272.
- Centers for Disease Control and Prevention. 2012. Diphtheria. in Epidemiology and Prevention of Vaccine-Preventable Diseases (12th edition), Atkinson W, Hamborsky J, Wolfe S, et al. (eds). Washington, DC. URL: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/dip.pdf (external link). (accessed 10 May 2022)
- Baker M, Taylor P ,Wilson E. A case of diphtheria in Auckland: implications for disease control. New Zealand Public Health Report, 1998. 5(10): p. 73–6.
- Institute of Environmental Science and Research Ltd. 2016. Notifiable Diseases in New Zealand: Annual Report 2015 (ed.), Porirua, New Zealand: The Institute of Science and Environmental Research Ltd. URL: https://surv.esr.cri.nz/PDF_surveillance/AnnualRpt/AnnualSurv/2015/2015AnnualReportFinal.pdf (external link) (accessed 3 July 2020)
- Sears A, McLean M, Hingston D, et al. Cases of cutaneous diphtheria in New Zealand: implications for surveillance and management. New Zealand Medical Journal, 2012. 125(1350): p. 64-71.
- Weir R, Jennings L, Young S, et al. 2009. National Serosurvey of Vaccine Preventable Diseases. https://www.health.govt.nz/system/files/documents/publications/national-serosurvey-of-vaccine-preventable-diseases-may09.pdf (external link) (accessed 30 June 2020)
- Fine PE. Herd immunity: history, theory, practice. Epidemiologic Reviews, 1993. 15(2): p. 265-302.
- Fine P, Mulholland K, Scott J, et al. 2018. Community Protection, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- Smith JW. Diphtheria and tetanus toxoids. British Medical Bulletin, 1969. 25(2): p. 177-82.
- Ad-hoc Working Group. Susceptibility to diphtheria. The Lancet, 1978. 311(8061): p. 428–30.
- Strategic Advisory Group of Experts on Immunisation (SAGE). 2017 Diphtheria vaccine. Review of evidence on vaccine effectiveness and immunogenicity to assess the duration of protection ≥10 years after the last booster dose. Geneva. URL: https://www.who.int/immunization/sage/meetings/2017/april/presentations_background_docs/en/ (external link). (accessed 18 June 2020)
- Bowie C. Tetanus toxoid for adults--too much of a good thing. Lancet, 1996. 348(9036): p. 1185-6.
- Stark K, Barg J, Molz B, et al. Immunity against diphtheria in blood donors in East Berlin and West Berlin. Lancet, 1997. 350(9082): p. 932.
- McIntyre PB, Burgess MA, Egan A, et al. Booster vaccination of adults with reduced-antigen-content diphtheria, Tetanus and pertussis vaccine: immunogenicity 5 years post-vaccination. Vaccine, 2009. 27(7): p. 1062-6.
- Hammarlund E, Thomas A, Poore EA, et al. Durability of Vaccine-Induced Immunity Against Tetanus and Diphtheria Toxins: A Cross-sectional Analysis. Clinical Infectious Diseases, 2016. 62(9): p. 1111-1118.
- McCormack PL. Reduced-antigen, combined diphtheria, tetanus and acellular pertussis vaccine, adsorbed (Boostrix®). A review of its properties and use as a single-dose booster immunization. Drugs, 2012. 72(13): p. 1765-1791.
- Havers FP, Moro PL, Hunter P, et al. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: updated recommendations of the Advisory Committee on Immunization Practices – United States, 2019. MMWR: Morbidity and Mortality Weekly Report, 2020. 69(3): p. 77-83.
- Australian Technical Advisory Group on Immunisation. 2018. Australian Immunisation Handbook (ed.), Canberra: Australian Government Department of Health. URL: https://immunisationhandbook.health.gov.au/ (external link) (accessed October 2019)
- Stratton KR, Howe CJ ,Johnston RB, Jr. Adverse events associated with childhood vaccines other than pertussis and rubella. Summary of a report from the Institute of Medicine. JAMA, 1994. 271(20): p. 1602-5.