On this page
Key information
|
Mode of transmission |
Airborne spread or by direct contact with nasal or throat secretions of cases. Measles is one of the most highly communicable of all infectious diseases (R0=12–18). |
|---|---|
|
Incubation period |
About 10 days but may be 7–18 days from exposure to onset of fever and 14 days (7-21 days) to onset of rash. The incubation period may be longer in those given IG after exposure. |
|
Period of communicability |
From 4 days before to 4 days after rash onset, counting the day of rash onset as day 0 (ie. a total of 9 days). |
|
Incidence and burden of disease |
New Zealand was declared free of endemic measles in 2017. Outbreaks continue to occur through imported cases, as occurred in 2019. To prevent recurrent outbreaks of measles, 95 percent of the population must be immune. |
|
Funded vaccine |
MMR (Priorix) is a live attenuated vaccine. |
|
Dose, presentation, route |
0.5 mL per dose after reconstitution. Pre-filled syringe and glass vial. The vaccine must be reconstituted prior to injection. Intramuscular or subcutaneous injection. |
|
Funded vaccine indications and schedule |
Children at ages 12 months and 15 months. Adults who are susceptible to one or more of measles, mumps and rubella. This includes all adults born in New Zealand from 1 January 1969 without two documented doses of measles-containing vaccine received after age 12 months. For (re)vaccination following immunosuppression (if the individual is immunocompetent enough to safely receive the vaccine). |
|
Recommended |
All adults born since January 1969 should be up to date with two doses of MMR or have evidence of immunity to all three vaccine components. It is particularly important for health care workers, individuals who work with children, armed forces personnel, staff of correctional facilities, long-term care facilities and immigration/refugee centres and laboratory staff. All vaccine-eligible travellers, particularly to high-risk countries. Some adults born overseas before 1969 may also be susceptible to measles, mumps or rubella. |
|
Vaccine effectiveness |
Measles vaccines are around 95 percent effective after 1 dose and 99 percent effective after two doses. |
|
Duration of protection |
Two doses are anticipated to provide lifelong protection. Protection is best achieved through herd immunity from high immunisation coverage. |
|
Contraindications |
MMR is contraindicated for immunocompromised individuals and in pregnancy. Priorix is contraindicated for anaphylaxis to neomycin. |
|
Precautions and special considerations |
See section 12.6 for cautions around receipt of blood products and other live vaccines, and other precautions. |
|
Potential responses to vaccine |
MMR is generally well tolerated. Fever and rash 6–12 days after vaccination. Salivary gland swelling and joint pain is possible due to mumps and rubella components. |
|
Public health measures |
Notify the local medical officer of health immediately on suspicion of wild-type measles. Promote immunisation to susceptible individuals. |
|
Post-exposure prophylaxis |
Management of contacts of measles cases should be discussed with the medical officer of health. (see section 12.8) |
12.1. Virology
The measles virus is an RNA virus, from the genus Morbillivirus, in the family Paramyxoviridae. Humans are the only natural host for the measles virus. The virus has a survival time of around two hours and is rapidly inactivated by sunlight, heat and extremes of pH.[1]
12.2. Clinical features
Measles is transmitted by airborne spread as well as direct contact with infectious droplets. It is one of the most highly communicable of all infectious diseases, with an approximate basic reproductive number of 12–18 in high-income countries[2] (see section 1.2.1). There is a prodromal phase with fever, conjunctivitis, coryza, cough and Koplik’s spots on the buccal mucosa. Other symptoms, such as anorexia, diarrhoea (especially in infants) and generalised lymphadenopathy, may also occur during this phase. The characteristic maculopapular rash classically appears first behind the ears three to five days after the first symptoms, before spreading downwards to the neck, trunk and the rest of the body. The number of lesions/spots generally increase in the first two to three days before starting to fade. The patient is most unwell during the first day or two after the appearance of the rash. Fever is always present at the time of rash onset and may peak around this time (generally over 39°C) before gradually decreasing. (For further details see the Communicable Disease Control manual)
(external link)The incubation period is about 10 days, but may be 7–18 days, from exposure to onset of fever, and about 14 days, but may be 7–21 days, from exposure to onset of rash. Measles is highly infectious from four days before to four days after rash onset, counting the day of rash onset as day zero. Incubation may be longer in those given IG after exposure or in infants with residual maternal antibody and can present as a modified, attenuated measles with a mild prodrome and a sparse discrete rash of short duration.[3]
Complications are common, occurring in 10 percent of cases, and include otitis media, pneumonia, croup and diarrhoea. Encephalitis has been reported in 1 in every 1,000 cases, of whom some 15 percent die and a further 25–35 percent are left with permanent neurological damage. Other complications of measles include bronchiolitis, sinusitis, myocarditis, corneal ulceration, mesenteric adenitis, hepatitis and idiopathic thrombocytopenic purpura (ITP or immune thrombocytopenia).
Measles infection causes acute immune suppression of the cellular and humoral immunity that leads to the depletion of immunological memory and antibody repertoire. This loss in immunity increases the long-term risk of further infections requiring medical treatment.[4, 5, 6, 7, 8] Although there are potential implications for long term effects on immune memory of individuals who have had measles, currently, there is no evidence to recommend re-immunisation.
Sub-acute sclerosing panencephalitis (SSPE) is a rare but fatal degenerative central nervous system disease resulting from persistent measles virus infection. It typically occurs 7–11 years after wild-type measles infection, at an estimated rate of 4–11 per 100,000 measles cases with higher incidence if measles occurs before 2 years of age.[9] Recent literature shows that SSPE is under-reported or under-diagnosed in the US.[10] Cases have occurred following undiagnosed measles or clinically mild disease, particularly when immunisation coverage has been low or where infants too young to have been immunised have acquired the infection while travelling to endemic regions.[11, 12]
The case-fatality rate for reported cases of measles in the US is 1–3 per 1,000.[9] Pneumonia is responsible for approximately 60 percent of deaths, more commonly in young patients. Measles is particularly severe and has a much higher case-fatality rate in the malnourished children with vitamin A deficiency, which is further exacerbated by diarrhoea; in patients with defective cell-mediated immunity, who may develop giant cell pneumonia or encephalitis without evidence of rash;[9] and in pregnant women.[9] Measles during pregnancy can cause miscarriage, stillbirth and preterm delivery.[1]
Measles is also serious in healthy children: over half of all the children who died from measles in the UK between 1970 and 1983 were previously healthy.[13 (external link)] No other conditions were reported as contributing to the death of seven people who died from measles in the 1991 New Zealand epidemic.
12.3. Epidemiology
12.3.1. Global burden of disease
12.3.1. Global burden of disease
Mortality and morbidity
From 2000 to 2016, the annual reported measles incidence decreased by 75 percent worldwide, from 146 to 35 cases per million population, due to increased vaccine coverage.[14] Annual worldwide estimated measles deaths was 89,780 in 2016, representing an 84 percent decline since 2000.[15]
However, there was a worldwide increase in disease during 2019. Over 1.3 million confirmed and suspected cases of measles were reported to WHO from 187/194 member states, resulting from multiple outbreaks, including in the previously measles-free Americas.[16]
Although measles mortality rates have fallen significantly,[17] measles remains an important vaccine-preventable cause of death among children throughout the world, particularly in low-income countries in Africa and Asia. The disease is highly infectious in non-immune communities, with epidemics occurring approximately every second year. During 2019, the countries with the highest number of cases were Philippines, Madagascar, India, Ukraine, the Nigeria and Brazil.[16] The incidence rate in Samoa was almost 10 times that observed in New Zealand (4525 versus 476 per million population). In Samoa, around 1.5 percent of cases died and one-third were hospitalised (there were 83 deaths, 5,707 cases and 1,868 hospitalised as of 20 January 2020).[18]
Measles elimination
When a country is verified by the Measles Regional Verification Commission as having eliminated measles, it means that the country interrupted transmission of the endemic strain of circulating measles virus for a period of 36 months. Importations of measles virus may have occurred during this period, but circulation of the imported strains of measles virus was interrupted within 12 months of the importation.[19]
In May 2012 the 194 member states of the World Health Assembly endorsed the Global Vaccine Action Plan 2011–2020,[20] which aimed to eliminate measles in at least four WHO regions by 2015 and in five WHO regions by 2020. In September 2016, the Region of the Americas was the first WHO region to be declared free of endemic measles and New Zealand was verified as having eliminated measles in 2017.
Disappointingly, no region has sustained measles elimination. Measles cases were reported to have climbed by 300 percent globally in the first three months of 2019.[21] For example, four European countries (Albania, Czechia, Greece and the UK) lost measles elimination status and the US reported it highest number of cases in 25 years.[22] Global coverage for the first measles vaccine dose has stalled at 85 percent and for the second doses at 67 percent, falling short of the 95 percent required to prevent outbreaks.[21 (external link)]
12.3.2. New Zealand epidemiology
12.3.2. New Zealand epidemiology
Measles vaccine was introduced in in New Zealand in 1969 and moved to a two-dose schedule (as a combined MMR vaccine) in 1992. Measles became a notifiable disease in 1996. The two-dose schedule at ages 15 months and 4 years was introduced in 2001 (see Appendix 1 for more information about the history of the Schedule) and was changed in 2020 to 12 months and 15 months following the 2019 outbreaks.
Historical holes in coverage, due to a combination of issues including historically low immunisation coverage in the childhood programme, unfounded vaccine safety concerns at the turn of the 21st century, changes to the schedule for MMR dose 2 from age 11 years to 4 years and compromised vaccine due to lack of adequate cold chain processes prior to 2004 have meant that young adults and adolescents (ages 15–30 years), in particular, are under immunised against measles.[23]
Prior to the introduction of two-dose MMR schedule, measles epidemics occurred in 1991 (the number of cases was estimated to be in the tens of thousands) and 1997 (when 2,169 cases were identified). In 2019, nine outbreaks occurred, six were linked to imported cases from the Philippines, Japan, Thailand, Australia and Singapore. In 2019, 2,213 cases were notified, of which 775 were hospitalised (ESR, 8 June 2020). The worst affected district was Counties Manukau, which had 1,174 cases, many of whom were of Māori and Pacific ethnicity, children too young to be immunised and unimmunised young adults.[24]
Importation of measles by non-immune people who had travelled overseas was also linked to the smaller measles outbreaks in New Zealand in 2009, 2011, 2014 and 2016 (see Figure 12.1; see also section 12.5.5).
Figure 12.1: Number of measles notifications by month reported, January 2009 to December 2019
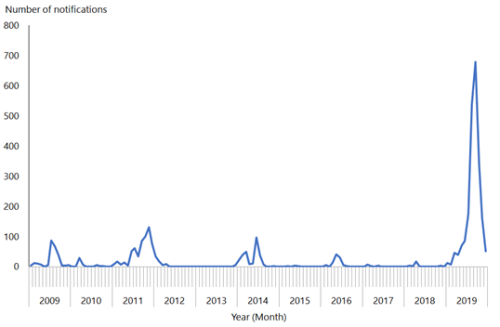
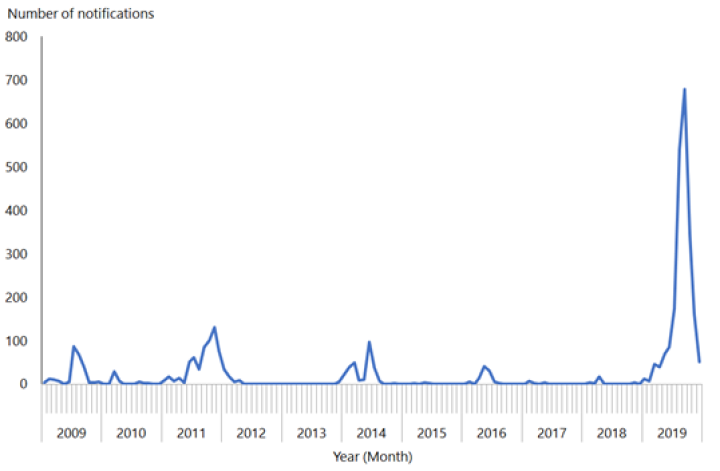
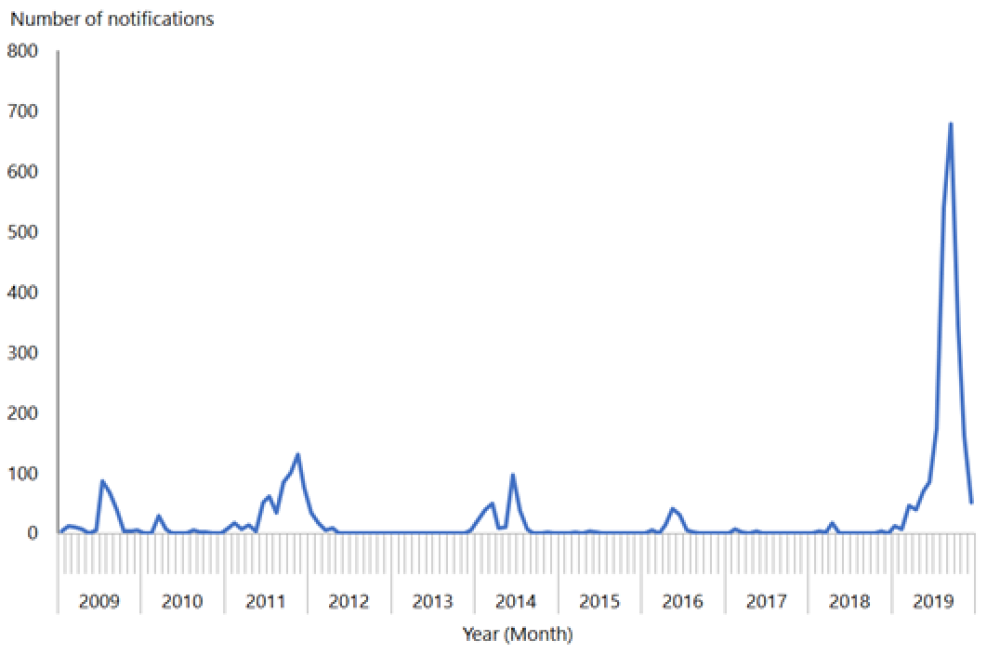
Source: ESR
To eliminate measles epidemics, modelling suggests that New Zealand needs to achieve a coverage level of 90 percent or greater for both doses of MMR.[25] If this coverage level is achieved and maintained in those aged 12 months to 50 years, elimination of measles can be maintained. In quarter ending 31 December 2019, the 5‑year-old immunisation coverage rate (ie, fully immunised with two doses of MMR) was 89 percent – nearing a target of 95 percent.
For details of measles notifications, refer to the most recent measles and notifiable disease reports from PHF Science (formerly ESR) (external link).
12.4. Vaccines
12.4.1. Available vaccines
12.4.1. Available vaccines
The measles vaccine is only available as one of the components of MMR. This vaccine is a freeze-dried preparation containing live attenuated measles, mumps and rubella viruses.
Funded vaccine
Each 0.5 mL dose of the reconstituted MMR (Priorix, GSK) contains:
- not less than 103.0 CCID50 of the attenuated line of Schwarz strain measles, propagated in chick embryo tissue culture
- not less than 103.7 CCID50 of RIT 4385 mumps strain, derived from the Jeryl Lynn strain and propagated in chick embryo tissue culture
- not less than 103.0 CCID50 of the Wistar RA 27/3 rubella strains, propagated in MRC5 human diploid cells
- lactose, amino acids supplement, mannitol, sorbitol and neomycin sulphate as excipients, and water for injection.
Other vaccines
M-M-R II (MSD) was the funded vaccine prior to the 1 July 2017 Schedule change. It contains a attenuated line of measles virus derived from Enders’ attenuated Edmonston strain; the Jeryl Lynn (B level) strain of mumps virus; and the Wistar RA 27/3 strain of live attenuated rubella virus.
Two quadrivalent measles, mumps, rubella and varicella vaccines (MMRV: see chapter 24) are also registered but not currently available in New Zealand:
- Priorix-Tetra (GSK) contains n the Schwarz measles, RIT 4385 mumps, Wistar RA 27/3 rubella and Oka/Merck varicella-zoster virus strains.
- ProQuad (MSD) contains Enders’ attenuated Edmonston (Moraten) measles virus strain, Wistar RA 27/3 rubella virus, Jeryl Lynn mumps virus and live varicella virus vaccines (Oka/Merck).
12.4.2. Efficacy and effectiveness
12.4.2. Efficacy and effectiveness
Measles vaccines are highly efficacious, and immunisation programmes have controlled measles to the point of elimination in many populations.[26] Outbreaks and epidemics continue to occur where low immunisation rates and/or sufficient numbers of susceptible members of communities are present. A 2012 Cochrane review of the safety and effectiveness of MMR concluded that a single dose of MMR is at least 95 percent effective in preventing clinical measles and 92 percent effective in preventing secondary cases among household contacts aged 6 months and older.[27] This was a systematic review of clinical trials and studies, which involved approximately 14.7 million children.
Seroconversion to all three viruses of MMR occurs in 85–100 percent of recipients. ‘Primary vaccine failure’ refers to the lack of protective immunity despite vaccination. It is due to failure of the vaccine to stimulate an immune response. This occurs in 5–10 percent of recipients after the first dose and is rare after a second dose. More than 99 percent of people who receive two MMR doses (given at least four weeks apart, with the first dose given after age 12 months) develop serologic evidence of immunity to measles.[9] Two doses are required for measles control and elimination in populations.[9] The second MMR dose is not a booster: it is given to increase vaccine efficacy to 98 percent and address primary vaccine failure.
Measles vaccination may have nonspecific effects, reducing mortality from other infectious diseases. Infection with the measles virus causes immune memory loss and predispose people to opportunistic infections for more than three years.[5, 7, 8] Population-level data from the UK, US and Denmark indicates that when measles was common, measles virus infections could have been implicated in as many as half of all childhood deaths from infectious disease.[8] The reduction in measles infections is suggested to be the main factor in reducing overall childhood infectious disease mortality after the introduction of vaccination.
Duration of immunity
Even though antibody levels decline over time, secondary vaccine failure (ie, vaccine failure due to waning of protective immunity) has been documented for measles but remains rare.[28] There were three cases in the recent New Zealand outbreak with documented secondary vaccine failure but no transmission was recorded from these cases. Breakthrough cases, as a result of secondary vaccine failure, appear to be attenuated and less likely to be hospitalised.[29]
In Finland in 1982 a cohort was recruited at the start of the national MMR vaccination programme to study the persistence of vaccine-induced antibodies. By the mid-1990s Finland had eliminated measles, mumps and rubella, and there was little opportunity for natural boosting to occur. The follow-up of this cohort has shown that while antibodies wane over time, 20 years after the second MMR dose, 95 percent of people remained seropositive for measles.[30] The antibody avidity also decreased by 8 percent for measles.[31] Waning of both the concentration and the avidity of antibodies might contribute to measles infections occurring in individuals who have received two doses of MMR. Waning in immunity was suggested in a small proportion of vaccinated cases (less than 1 percent) in the 31–42 year age group reported in an outbreak in Berlin.[32 (external link)]
12.4.3. Transport, storage and handling
12.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
Store at +2°C to +8°C. Do not freeze.
MMR must be reconstituted only with the diluents supplied by the manufacturer. Use MMR as soon as possible after reconstitution. If storage is necessary, reconstituted vaccine can be stored at +2°C to +8°C for up to eight hours.
12.4.4. Dosage and administration
12.4.4. Dosage and administration
The dose of MMR is all of the reconstituted vaccine (approximately 0.5 mL) administered by intramuscular injection, or subcutaneous injection if indicated (see section 2.2.3).
Co-administration with other vaccines
MMR can be given concurrently with other vaccines, as long as separate syringes are used, and the injections are given at different sites. If not given concurrently, live vaccines should be given at least four weeks apart. (See also section 2.2.7 for information about multiple injections at the same visit.) Note that TST/Mantoux testing or interferon-gamma release assay for tuberculosis must be deferred by at least four weeks after MMR if not given concurrently (see section 12.6.2).
Interchangeability
The two brands of MMR (Priorix and M-M-RII) may be used interchangeably for completion of a course.[33]
12.5. Recommended immunisation schedule
Table 12.1: Recommended MMR vaccination schedule
Table 12.1: Recommended MMR vaccination schedule
|
|
Schedule |
|---|---|
|
Usual childhood schedulea |
2 doses: at ages 12 months and 15 months |
|
Catch-upb for children, adolescents and adults |
2 doses: at least 4 weeks apart |
|
a. If MMR is given to children aged under 12 months, 2 further MMR doses are still required at age 12 months (given at least 4 weeks since the previous dose) and 15 months. b. For those born from 1 January 1969 who do not have documented evidence of two doses of a MMR-containing vaccine given after age 1 year, or who do not have serological evidence of protection for measles, mumps and rubella. See section 12.5.2. |
|
12.5.1. Usual childhood schedule
12.5.1. Usual childhood schedule
A two-dose MMR vaccination schedule (with appropriate documentation) is recommended irrespective of a history of measles, mumps or rubella infection or measles immunisation. A clinical history does not reliably indicate immunity. Positive serology for measles cannot be used as a proxy for mumps or rubella immunity. There are no known ill effects from vaccinating children, even if they have had serologically confirmed infection with any of the viruses.
Measles vaccine is recommended as MMR at age 12 and 15 months. Two doses of measles vaccine are recommended because nearly all the approximately 5 percent who fail to be protected by the first dose will be protected by the second.[34] The second dose of measles vaccine can be given as soon as four weeks after the first dose.
MMR vaccination when aged under 12 months
A dose of MMR (called MMR0 [zero]) may be recommended for infants aged 4 months to under 12 months during measles outbreaks for pre-exposure prophylaxis (for post-exposure prophylaxis and HNIG use see section 12.8). These children still require a further two doses of MMR at ages 12 months (given at least four weeks after previous dose) and 15 months because their immune response and long-term protection from measles is less when the vaccine is given at a younger age.[35, 36] Any recommendations in this regard will be made by the local medical officer of health and Health New Zealand | Te Whatu Ora based on local epidemiology.
The first dose of MMR is scheduled and recommended at age 12 months, if infants are given MMR from age 11 months in other circumstances, they are considered to have been given MMR1 and therefore only need one further dose, ideally given at age 15 months. To optimise early protection, dose two can be given from age 12 months, at least four weeks later. For toddlers and children older than 15 months, give both doses of MMR four weeks apart.
Note:
- For infants born to mothers who received immunomodulatory biologic agents in pregnancy, see precautions section 12.6.2 and section 4.2.5.
- Some immigrant children may have received a measles-containing vaccine when aged under 12 months.
12.5.2. Catch-up
12.5.2. Catch-up
Two doses of documented MMR (at least four weeks apart) are recommended and funded for any child, adolescent or adult who is known to be susceptible to one or more of the three diseases.
A second dose of MMR should be given at least four weeks after MMR1 to all children who are older than 15 months but have not yet received their second MMR dose (ie, those who would have been due their second dose at age 4 years).
Adults born from 1 January 1969
All individuals born in 1969 or later who have documented evidence of two doses of MMR given after age 12 months or who have serological evidence of protection for measles are considered immune to measles.
Some adults may have received one dose of measles-containing vaccine and one dose of MMR or MR during one of the catch-up campaigns (eg, the 1997 campaign, when all those aged up to 10 years were offered MMR, or from international schedules). They will have therefore received the recommended two doses of measles vaccine, but only one of mumps and rubella vaccines. While the main reason for a two-dose MMR schedule is to protect against measles, two doses of all three antigens is recommended and funded. These individuals can receive a second dose of MMR (ie, a third dose of measles vaccine) without any concerns. It is important that women of childbearing age are immune to rubella (see chapter 21).
All persons born from 1 January 1969 with only one documented dose of prior MMR should receive a further dose of MMR; if there are no documented doses of prior MMR or documented evidence of immunity, then two doses should be administered, at least four weeks apart.
Occupational risk groups
Certain occupational groups are at increased risk of exposure to measles; particularly those who travel frequently, such as military personnel and cruise ship staff and aircrew (in this case MMR is funded for those eligible for New Zealand health services). In some cases, positive serology is required as a proof of immunity. In those who are deemed serologically negative, although two doses of measles-containing vaccine are documented, give a further dose of MMR. See Table 4.9 for details of relevant occupational groups.
For occupational protection, either serology or an additional dose of MMR (dose 3) can be used. There are no additional safety concerns around giving further MMR doses.
12.5.3. Immunocompromise
12.5.3. Immunocompromise
Contacts of immunocompromised individuals
In general, MMR is contraindicated in immunocompromised individuals (see section 4.2.5). These people can be partially protected from exposure to infection by ensuring that all their close contacts, including hospital staff and family members, are fully immunised (this is funded), including hospital staff and family members, through cocoon strategies. There is no risk of transmission of MMR vaccine viruses from a vaccine recipient to an immunocompromised individual. See section 12.7.2.
See also ‘Vaccination of close contacts of immunocompromised individuals’ in section 4.3.1 for general vaccination information for contacts of immunocompromised individuals.
(Re)vaccination before or following immunosuppression
MMR is funded to be given at least 28 days before planned immunosuppression.
For vaccination or revaccination following immunosuppression (funded), it is important to be sure that the individual is immunocompetent enough to safely receive the vaccine.
HIV infection
Discuss vaccination of individuals with HIV infection with their specialist (see ‘HIV infection’ in section 4.3.12).
Receipt of MMR, on time at age 12 and 15 months, is recommended for asymptomatic children who are not severely immunocompromised. MMR is recommended for all HIV-positive individuals aged from 12 months, whether they are symptomatic or asymptomatic, if their CD4+ lymphocyte level is over 15 percent or CD4+ lymphocyte count is at least 200 cells/mm3. Administration of MMR with CD4+ counts below these recommended levels has been associated with vaccine-related pneumonitis (from the measles component).[9]
12.5.4. Pregnancy and breastfeeding
12.5.4. Pregnancy and breastfeeding
MMR is contraindicated during pregnancy. Pregnancy should be avoided for four weeks after MMR vaccination.[1, 9, 34]
After delivery
If MMR and Rhesus anti-D IG are required after delivery, both the vaccine and anti-D IG may be given at the same time, in separate sites with separate syringes. The vaccine may be given at any time after the delivery. Anti-D IG does not interfere with the antibody response to the vaccine, but whole blood transfusion does inhibit the response in up to 50 percent of vaccine recipients (see section A6.4.1).
MMR can be given to breastfeeding women.
(See also sections 4.1.3, 12.6.1 and 21.5.3.)
12.5.5. Travel
12.5.5. Travel
International travel is an important factor in reintroducing measles into New Zealand. To avoid importing measles to New Zealand, vaccination with MMR is recommended for all children aged 12 months and older and adults travelling overseas if they have not previously been adequately vaccinated (see Appendix 2 for planning catch-up immunisations). Infants aged from 4 months can receive a dose of MMR if they are travelling to a country with an active measles outbreak. These children will still require two doses from age 12 months to be fully immunised.
Measles remains endemic in many countries, including areas in Europe, Asia, the Pacific and Africa, and outbreaks are ongoing worldwide. A high rate of measles incidence was reported in the Philippines during 2019 and early 2020.[37] During January to October 2019, 52 (64 percent) out of 81 imported measles cases in the US were residents returning from travel abroad and 73 (90 percent) were unvaccinated or had unknown vaccination status. Eight outbreaks (85 percent of cases of the resulting 1,259 cases) were associated with unvaccinated close-knit populations, such as the Orthodox Jewish community in New York; these began from two single imported cases.[38] Travel was also linked to the measles outbreaks in New Zealand in 2011, 2014, 2016 and 2019.
12.6. Contraindications and precautions
See also section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 for general vaccine contraindications.
12.6.1. Contraindications
12.6.1. Contraindications
The general contraindications that apply to all immunisations are relevant to MMR (eg, children with an acute febrile illness should have their immunisation deferred).
Anaphylaxis following a previous dose of MMR or any of the vaccine components is a contraindication to a further dose of MMR. Individuals who have anaphylaxis after receiving MMR should be serologically tested for immunity and referred to, or discussed with, a specialist if they are non-immune to rubella or measles.
MMR is contraindicated for:
- those with proven anaphylaxis to the vaccine or vaccine component (eg, neomycin)
- immunocompromised individuals (ie, those with significantly impaired cell-mediated immunity, such as those with untreated malignancy, type 1 interferon receptor (IFNAR) signalling pathway defects, or altered immunity as a result of drug therapy, including high-dose steroids, or receiving high-dose radiotherapy) (see section 4.2.5)
- individuals who have received another live vaccine, including BCG, within the previous 4 weeks unless given concurrently (for BCG, see chapter 23)
- pregnant women – pregnancy should be avoided for four weeks after immunisation[1, 9] see section 21.5.3)
- individuals who have received IG or a blood transfusion during the preceding 11 months (see Table A6.1 in Appendix 6 for the length of time to defer measles vaccine after specific blood products)
- those with severe immune deficiency from HIV, because vaccine-related pneumonitis (from the measles component) has been reported[9] – discuss vaccination of individuals with HIV infection with their specialists.
Note: Inadvertent immunisation with a rubella-containing vaccine in early pregnancy is no longer considered an indication for termination of pregnancy. There have been no cases of teratogenic damage from vaccine virus despite intensive surveillance in the US, the UK and Germany.[39]
12.6.2. Precautions
12.6.2. Precautions
Children with a history of seizures should be given MMR, but the parents/guardians should be warned that there may be a febrile response. In the case of children with current ITP, the timing of vaccination should be discussed with their specialist.
Women of childbearing age should be advised to avoid pregnancy for the next four weeks after MMR vaccination (see section 21.5.3).[1, 9]
Measles vaccination may temporarily suppress tuberculin skin test (TST/Mantoux) reactivity or interferon-gamma release assay (IGRA). If these are required, a TST should be placed or blood sample taken for IGRA on the same day as MMR vaccination or be postponed for at least four weeks after vaccination.[34] TST is not a prerequisite for measles vaccination. An individual with active TB should be established on treatment before administering MMR.
For infants aged under 12 months, please discuss immunomodulatory therapies taken during pregnancy with infant’s mother or specialist, or contact IMAC before administration of MMR0. See section 4.2.5.
Interferon alpha receptor deficiencies
IFNAR deficiencies can increase the risk of severe reaction to some live viral vaccines, such as MMR. These are extremely rare immune disorders that can result in a defective response to certain viruses, including measles and COVID-19.[40] If an older sibling or relative had a severe illness following MMR this should be discussed with immunisation specialists. If a child did not develop a serious illness in response to their first dose of MMR, it appears unlikely that they will have a serious reaction to their second MMR vaccine due to undiagnosed IFNAR1 deficiency. For more information see section 4.3.2.
12.6.3. Egg allergy
12.6.3. Egg allergy
The measles and mumps components of the MMR are manufactured in chick embryo cell culture, so there may be trace amounts of egg protein in the vaccine. Egg allergy, including anaphylaxis, is not a contraindication to measles-containing vaccines. Various studies have confirmed that children with egg allergy can be vaccinated safely.[34, 41, 42] Other components of the vaccine may be responsible for allergic reactions.[43] Individuals with egg allergy may therefore be safely vaccinated in primary care.[44]
12.7. Potential responses and AEFIs
12.7.1. Potential responses
12.7.1. Potential responses
Some children, around 5–15 percent, may experience fever of 39.4°C or more between 5 and 12 days after immunisation that generally lasts one to two days and is associated with a strong measles antibody response.[34, 45] Transient rash, often with atypical distribution, can occur in approximately 5 percent of vaccine recipients at the same interval post-vaccination: these individuals are not infectious to others.[34] Fevers, rashes and other systemic symptoms can occur coincidentally after vaccination due to common childhood infections and are not caused by the vaccine.[46]
Serological tests or PCR can be expected to be positive if performed during this time, so testing should not be routinely performed. During an outbreak, genotyping the sample can help to identify vaccine strain versus wild type disease.
The mumps vaccine may produce parotid and/or submaxillary swelling in about 1 percent of vaccine recipients, most often 10–14 days after immunisation.[47] The rubella vaccine can cause a mild rash, fever, joint pain and lymphadenopathy between one and three weeks after immunisation.[9, 48] There were no persisting sequelae associated with the administration of 3 million doses of MMR to 1.5 million children in Finland.[46, 49]
12.7.2. AEFIs
12.7.2. AEFIs
Temporally related reactions, including febrile seizures, nerve deafness, aseptic meningitis, encephalitis, rash, pruritus and purpura, may follow immunisation rarely; however, causality has not been established.[50]
Vaccine virus transmission
MMR vaccine viruses are regarded as being non-transmissible from vaccine recipients. Two historical, poorly documented case of transmission (of a rubella and a mumps vaccine strain) were reported from a vaccine that is no longer in production.[51] Following immunisation with both measles and rubella vaccines, live virus has been isolated rarely from pharyngeal secretions.[39, 52] There have been no confirmed cases of disease transmission from MMR vaccine viruses.
Idiopathic thrombocytopenic purpura
MMR is the only childhood vaccine with an elevated risk of ITP, which occurs in 1 in 25,000 to 40,000 people, 15 days to six weeks after immunisation.[9] ITP is mild and transient, resolving within six months in 93 percent of cases.[1, 53] If ITP occurs, measles, mumps and rubella serology should be measured, and if the individual is immune to all three infections, a second dose is not required. However, if the individual is susceptible to any of the three infections, a second dose should be administered.[54, 55, 56] The risk of thrombocytopenia is higher after the first dose of vaccine than after the second dose and is much rarer after vaccination than after wild-type infection.[9 (external link)]
12.7.3. Adverse outcomes not linked to MMR
12.7.3. Adverse outcomes not linked to MMR
Over three decades, multiple published epidemiological studies in the UK,[57] Finland[58] and elsewhere[27] have confirmed that there is no link between MMR and the development of autism in young children (see section 3.2.4 for further discussion on this issue).
12.8. Public health measures
It is a legal requirement that all cases of measles be notified immediately on suspicion to the local medical officer of health – do not wait for a laboratory confirmation as this can delay contact tracing and allow outbreaks to become established if disease is confirmed.
A single case of measles should be considered an outbreak and result in an appropriate outbreak response. Practitioners should have a low index of suspicion for notification, and all suspected clinical cases should be self-isolated immediately and notified to the medical officer of health. It is important that the diagnosis be laboratory confirmed, as many viral infections can mimic measles.
For details of public health control measures, case definitions and management see the measles chapter in the Communicable Disease Control Manual measles chapter.
12.8.1. Post-exposure prophylaxis
12.8.1. Post-exposure prophylaxis
Management of contacts of a measles case should be discussed with the local medical officer of health.
MMR vaccination
A single dose of MMR when given to an unvaccinated person, from age 6 months, within 72 hours of first contact with an infectious person may reduce the risk of developing disease as post exposure prophylaxis.[1] If there is doubt about vaccination status, MMR should still be given. MMR will not exacerbate the symptoms of measles if a person is already incubating the disease, but in these situations, any measles-like illness occurring shortly after vaccination is likely to be due to infection. Note, for post-exposure prophylaxis of infants aged under 6 months, give HNIG (see below) as a priority, not MMR0 (See Table A6.1 for spacing of MMR after HNIG). Pre-exposure MMR vaccination can be given from age 4 months.
If MMR is not given within 72 hours of first exposure, it should still be offered at any later interval to provide protection from future exposures, unless the vaccine is contraindicated.
Most individuals born prior to 1969 will have measles immunity, either through wild-type infection or measles vaccination. Individuals who were born in countries with limited circulating measles prior to 1969 can be considered for post-exposure prophylactic MMR vaccination in the case of a community outbreak. Seek advice from IMAC prior to vaccinating adults born prior to 1960.
Immunoglobulin prophylaxis for contacts
Human normal immunoglobulin (HNIG; Normal Immunoglobulin-VF) is recommended for measles-susceptible individuals in whom the vaccine is contraindicated (see section 12.6) and susceptible pregnant contacts. For these individuals, HNIG is given to attenuate disease and should be given as soon as possible, up to a maximum of six days after exposure. All other susceptible contacts should be offered MMR as post-exposure prophylaxis (as described above).
HNIG provides no benefit to those who are already exhibiting symptoms of measles.
For details and eligibility see the Measles chapter, post-exposure prophylaxis in the Communicable Disease Control Manual available at www.tewhatuora.govt.nz/for-health-professionals/clinical-guidance/communicable-disease-control-manual/measles#contact-management. (external link)
For further details for infants under 6 months and immunocompromised children refer to the Starship Child Health guidelines (external link).
12.9. Variations from the vaccine data sheet
The vaccine data sheet recommends a single dose of MMR. However, as 2–5 percent of recipients fail to seroconvert after the first dose (see section 12.4.2), Health NZ | Te Whatu ora recommends and funds a second dose of MMR. Two doses are required for measles control and elimination; the second MMR dose is not a booster.[34]
The vaccine data sheet states that individuals who have experienced anaphylaxis after egg ingestion should be vaccinated with extreme caution, with adequate treatment for anaphylaxis on hand should such a reaction occur. However, various studies have confirmed that egg-allergic children can be vaccinated safely.[9, 41, 42] Health New Zealand | Te Whatu Ora recommends that individuals with egg allergy, including anaphylaxis, may be safely vaccinated in primary care (see section 12.6.3).
References
References
References
- Strebel P, Papania M, Gastañaduy P, et al. 2018. Measles Vaccine, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- Fine P, Mulholland K, Scott J, et al. 2018. Community Protection, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- Centers for Disease Control and Prevention. 2019. Measles. in Epidemiology and Prevention of Vaccine-Preventable Diseases. The Pink Book 13th edition. URL: https://www.cdc.gov/vaccines/pubs/pinkbook/meas.html (external link). (accessed 6 April 2020)
- Laksono BM, de Vries RD, Verburgh RJ, et al. Studies into the mechanism of measles-associated immune suppression during a measles outbreak in the Netherlands. Nat Commun, 2018. 9(1): p. 4944.
- Mina MJ, Kula T, Leng Y, et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science, 2019. 366(6465): p. 599-606.
- Petrova VN, Sawatsky B, Han AX, et al. Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci Immunol, 2019. 4(41).
- Gadroen K, Dodd CN, Masclee GMC, et al. Impact and longevity of measles-associated immune suppression: a matched cohort study using data from the THIN general practice database in the UK. BMJ Open, 2018. 8(11): p. e021465.
- Mina MJ, Metcalf CJ, de Swart RL, et al. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science, 2015. 348(6235): p. 694-9.
- American Academy of Pediatrics. 2018. Measles. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, et al. (eds). Elk Grove Village, IL. p. 537-550. URL: https://redbook.solutions.aap.org/redbook.aspx (external link). (accessed 3 July 2020)
- Wendorf KA, Winter K, Zipprich J, et al. Subacute sclerosing panencephalitis: the devastating measles complication that might be more common than previously estimated. Clinical Infectious Diseases, 2017. 65(2): p. 226-232.
- Bellini WJ, Rota JS, Lowe LE, et al. Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. Journal of Infectious Diseases, 2005. 192(10): p. 1686-93.
- Pittet LF ,Posfay-Barbe KM. Increasing incidence of subacute sclerosing panencephalitis in infants: a collateral effect of under-vaccination. Clinical Microbiology and Infection, 2020. (pre-print).
- Miller C. Deaths from measles in England and Wales, 1970–83. British Medical Journal, 1985. 290(6466): p. 443–4.
- World Health Organization. Measles vaccines: WHO position paper, April 2017 - Recommendations. Vaccine, 2019. 37(2): p. 219-222.
- World Health Organization. 2018 Measles. WHO; 2018 [updated April 2018 ]; URL: https://www.who.int/immunization/diseases/measles/en/ (external link). (accessed November 2019)
- World Health Organization. Measles and Rubella Surveillance Data.: WHO; [updated 13 March 2020]; URL: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/measles_monthlydata/en/ (external link) (accessed 25 April 2020)
- GBD 2015 Child Mortality Collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 2016. 388(10053): p. 1725–74.
- World Health Organization ,UNICEF. 2020 Measles outbreak in the Pacific - Situation report no. 11. URL: https://www.who.int/docs/default-source/wpro---documents/dps/outbreaks-and-emergencies/measles-2019/20200122-measles-pacific-who-unicef-sitrep-11.pdf?sfvrsn=9e1851f5_2 (external link) [PDF]. (accessed 3 July 2020)
- World Health Organization. Framework for verifying elimination of measles and rubella. Weekly Epidemiological Record, 2013. 88(9): p. 89-99.
- World Health Organization. 2013. Global Vaccine Action Plan 2011–2020 (ed.), Geneva: World Health Organization. URL: https://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/ (external link) (accessed 6 November 2019)
- World Health Organization. 2019 New measles surveillance data for 2019.: WHO; 2019 [updated April 2019]; URL: https://www.who.int/immunization/newsroom/measles-data-2019/en/ (external link). (accessed 3 July 2020)
- World Health Organization. 2019 More than 140,000 die from measles as cases surge worldwide (Press release). World Health Organization: Joint News Release. 5 December 2019URL: https://www.who.int/news-room/detail/05-12-2019-more-than-140-000-die-from-measles-as-cases-surge-worldwide (external link). (accessed 3 July 2020)
- Reynolds G, Dias C, Thornley S, et al. Analysis of the Auckland 2014 measles outbreak indicates that adolescents and young adults could benefit from catch-up vaccination. New Zealand Medical Journal, 2015. 128(1422): p. 53-62.
- Institute of Environmental Science and Research. 2020 Measles report. Public Health Surveillance: ESR; Public Health Surveillance; 2020; URL: https://surv.esr.cri.nz/surveillance/WeeklyMeaslesRpt.php (external link). (accessed 10 February 2020)
- Roberts M, A Mathematical Model for Measles Vaccination. 2004: Unpublished report to the Ministry of Health, New Zealand.
- Zahraei SM, Gouya MM, Azad TM, et al. Successful control and impending elimination of measles in the Islamic Republic of Iran. Journal of Infectious Diseases, 2011. 204 Suppl 1(Suppl 1): p. S305-11.
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev, 2012(2): p. CD004407.
- Albertson JP, Clegg WJ, Reid HD, et al. Mumps Outbreak at a University and Recommendation for a Third Dose of Measles-Mumps-Rubella Vaccine - Illinois, 2015-2016. MMWR: Morbidity and Mortality Weekly Report, 2016. 65(29): p. 731-4.
- Gibney KB, Attwood LO, Nicholson S, et al. Emergence of attenuated measles illness among IgG positive/IgM negative measles cases, Victoria, Australia 2008-2017. Clinical Infectious Diseases, 2019.
- Davidkin I, Jokinen S, Broman M, et al. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. Journal of Infectious Diseases, 2008. 197(7): p. 950-6.
- Kontio M, Jokinen S, Paunio M, et al. Waning antibody levels and avidity: implications for MMR vaccine-induced protection. Journal of Infectious Diseases, 2012. 206(10): p. 1542-8.
- Bitzegeio J, Majowicz S, Matysiak-Klose D, et al. Estimating age-specific vaccine effectiveness using data from a large measles outbreak in Berlin, Germany, 2014/15: evidence for waning immunity. Euro Surveillance, 2019. 24(17).
- Australian Technical Advisory Group on Immunisation (ATAGI). 2018. Measles. in Australian Immunisation Handbook. Canberra. URL: https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/measles (external link). (accessed 25 April 2020)
- American Academy of Pediatrics. 2024. Measles. in Red Book 2021-2024: Report of the Committee on Infectious Diseases (32nd Edition), Kimberlin D, Banerjee R, Barnett E, et al. (eds). p. 570-584. URL: https://publications.aap.org/redbook/book/347/chapter-abstract/5753982/Measles. (accessed 18 February 2025)
- Nic Lochlainn LM, de Gier B, van der Maas N, et al. Immunogenicity, effectiveness, and safety of measles vaccination in infants younger than 9 months: a systematic review and meta-analysis. Lancet Infectious Diseases, 2019. 19(11): p. 1235-1245.
- Nic Lochlainn LM, de Gier B, van der Maas N, et al. Effect of measles vaccination in infants younger than 9 months on the immune response to subsequent measles vaccine doses: a systematic review and meta-analysis. Lancet Infectious Diseases, 2019. 19(11): p. 1246-1254.
- Regional Office for the Western Pacific Region. 2019 Measles-Rubella Bulletin 2020. World Health Organization; 2019 [updated 20 May 2020]; URL: https://apps.who.int/iris/handle/10665/331240 (external link). (accessed 10 May 2022)
- Patel M, Lee AD, Clemmons NS, et al. National Update on Measles Cases and Outbreaks - United States, January 1-October 1, 2019. MMWR: Morbidity and Mortality Weekly Report, 2019. 68(40): p. 893-896.
- Reef SE ,Plotkin S. 2018. Rubella Vaccines, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- Bastard P, Hsiao K-C, Zhang Q, et al. A loss-of-function IFNAR1 allele in Polynesia underlies severe viral diseases in homozygotes. J. Exp. Med, 2022. 219(6).
- James JM, Burks AW, Roberson PK, et al. Safe administration of the measles vaccine to children allergic to eggs. New England Journal of Medicine, 1995. 332(19): p. 1262-6.
- Khakoo G ,Lack G. Recommendations for using MMR vaccine in children allergic to eggs. British Medical Journal, 2000. 320(7239): p. 929–32.
- Fox A ,Lack G. Egg allergy and MMR vaccination. British Journal of General Practice, 2003. 53(495): p. 801-2.
- Clark AT, Skypala I, Leech SC, et al. British Society for Allergy and Clinical Immunology guidelines for the management of egg allergy. Clinical and Experimental Allergy, 2010. 40(8): p. 1116-29.
- Carazo Perez S, Bureau A ,De Serres G. Post-immunisation fever and the antibody response to measles-containing vaccines. Epidemiology and Infection, 2018. 146(12): p. 1584-1592.
- Peltola H ,Heinonen OP. Frequency of true adverse reactions to measles-mumps-rubella vaccine. A double-blind placebo-controlled trial in twins. Lancet, 1986. 1(8487): p. 939-42.
- Rubin S. 2018. Mumps Vaccines, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- American Academy of Pediatrics. 2018. Rubella. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, et al. (eds). Elk Grove Village, IL. URL: https://redbook.solutions.aap.org/redbook.aspx (external link). (accessed 3 July 2020)
- Peltola H, Patja A, Leinikki P, et al. No evidence for measles, mumps, and rubella vaccine-associated inflammatory bowel disease or autism in a 14-year prospective study. Lancet, 1998. 351(9112): p. 1327-8.
- American Academy of Pediatrics. 2018. Mumps. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, et al. (eds). Elk Grove Village, IL. URL: https://redbook.solutions.aap.org/book.aspx?bookid=2205 (external link). (accessed 3 July 2020)
- Wolf JE, Eisen JE ,Fraimow HS. Symptomatic rubella reinfection in an immune contact of a rubella vaccine recipient. Southern Medical Journal, 1993. 86(1): p. 91-3.
- Morfin F, Beguin A, Lina B, et al. Detection of measles vaccine in the throat of a vaccinated child. Vaccine, 2002. 20(11-12): p. 1541-3.
- O'Leary ST, Glanz JM, McClure DL, et al. The risk of immune thrombocytopenic purpura after vaccination in children and adolescents. Pediatrics, 2012. 129(2): p. 248-55.
- Beeler J, Varricchio F ,Wise R. Thrombocytopenia after immunisation with measles vaccines: review of the vaccine adverse events reporting system (1990 to 1994). Pediatric Infectious Disease Journal, 1996. 15(1): p. 88–90.
- Miller E, Waight P, Farrington CP, et al. Idiopathic thrombocytopenic purpura and MMR vaccine. Archives of Disease in Childhood, 2001. 84(3): p. 227-9.
- Stowe J, Kafatos G, Andrews N, et al. Idiopathic thrombocytopenic purpura and the second dose of MMR. Archives of Disease in Childhood, 2008. 93(2): p. 182-3.
- Miller E. MMR vaccine: review of benefits and risks. Journal of Infection, 2002. 44(1): p. 1-6.
- Makela A, Nuorti JP ,Peltola H. Neurologic disorders after measles-mumps-rubella vaccination. Pediatrics, 2002. 110(5): p. 957-63.
- Public Health England. 2019. Guidelines on Post-Exposure Prophylaxis for measles. (ed.): Crown Copyright. URL: https://www.gov.uk/government/publications/measles-post-exposure-prophylaxis (external link) (accessed 21 October 2019)