On this page
Key information
|
Mode of transmission |
Spread by droplets generated by sneezing and coughing, by direct or indirect contact, or by the aerosol route. |
|---|---|
|
Incubation period |
Usually 1–3 days (range 1–7 days). |
|
Period of communicability |
From 1–2 days before symptoms start until about day 5 of illness; may be longer in young children and if immunocompromised. Asymptomatic spread is common. |
|
Incidence and burden of disease |
Influenza epidemics occur each year. The highest burden of disease is in the very young, the elderly, pregnant women, those with co-morbid conditions, people from low-income groups and Māori and Pacific ethnic groups. |
|
Funded vaccines |
|
|
Dose, presentation, route |
0.5 mL for adults and children 6 months of age and above Pre-filled syringe, needle attached Intramuscular injection, or deep subcutaneous injection (if indicated) |
|
Funded vaccine indications and recommended schedule |
1 dose is recommended and funded annually from 1 April for:
Children aged under 9 years who have not previously received influenza vaccine require 2 doses 4 weeks apart (funded for children with eligible conditions). |
|
Recommended, unfunded |
Universally recommended for anyone age from 6 months, annually.
|
|
Vaccine effectiveness |
Depends on the match of the strains in the vaccine with circulating strains, the age of the individual and whether they have any underlying medical conditions. Vaccination can prevent disease or reduce severity. |
|
Precautions and special considerations |
There may be a small increased risk of fever and febrile convulsions with concomitant delivery of PCV13 and influenza vaccine in children aged 6 months to under 5 years. |
|
Potential response to vaccine |
Mild fever, headache, muscle aches, local swelling and mild pain at injection site. Children aged under 5 years are more likely than older children or adults to have a febrile reaction to influenza vaccine. |
11.1. Virology
Influenza viruses belong to the Orthomyxoviridiae family, and are classified into influenza virus types A, B and C. Influenza A virus subtypes are classified based on two surface antigens:
- haemagglutinin (H), responsible for cell surface attachment during infection
- neuraminidase (N), which potentiates the release of new virions from the cell.
Subtypes which have in the past caused pandemics include the influenza A H1N1, H2N2, H3N2 and H1N1pdm09 viruses, while the H3N2 and H1N1pdm09 viruses continue to cause epidemics as seasonal influenza viruses. Prior to 2020, two lineages of influenza B, B/Victoria and B/Yamagata, were associated with outbreaks and epidemics, and accounted for a significant proportion of the overall burden of influenza.[1] Although B/Victoria continues to contribute to the burden of influenza, with no detections since March 2020, it appears that the B/Yamagata strain is potentially, globally extinct.[2] Influenza C is associated with mild cases of upper respiratory infection.
11.1.1. Antigenic drift
11.1.1. Antigenic drift
Influenza A and B viruses undergo frequent small changes (mutations) in their segmented RNA genome over time. The mutations can occur in the coding regions responsible for H and N surface antigens. This ‘antigenic drift’ leads to the emergence of new antigenic variants or virus strains.
These new strains are described by the geographic site of isolation, laboratory number and year of isolation; for example, A/Hong Kong/4801/2014 (H3N2). Because of this ongoing antigenic drift, WHO reviews seasonal influenza virus vaccine formulations bi-annually.
11.1.2. Antigenic shift
11.1.2. Antigenic shift
New influenza A virus subtypes emerge periodically that have caused pandemics in humans. The new virus subtype has novel H and N surface antigens result from the mixing of genomic segments of two or more influenza A viruses. This is known as ‘antigenic shift’. Other possible mechanisms for the emergence of new influenza viruses are through the adaptation of avian influenza viruses to infect humans and the re-assortment of the genomic segments of multiple viruses (ie, human, avian and pig influenza viruses).
11.2. Clinical features
Influenza is contagious, with a reproductive number (R0) estimated at 1.4–4 (see section 1.2.1).[3] The virus is transmitted by respiratory droplets generated by sneezing and coughing that land directly on respiratory mucous membranes by aerosolised droplets or by direct or indirect contact (via contaminated hands or fomites).[3, 4, 5] The incubation period can range from one to seven days (average one to three days), during which time the virus replicates in the ciliated columnar epithelial cells of the upper and lower respiratory tract. An infected person is contagious from one to two days before symptoms start until about day five of the illness. Peak viral shedding occurs one to three days after the development of symptoms, diminishing to low levels by five days. Children shed more virus and remain infectious for longer than adults.
There is a wide range of symptoms, from asymptomatic to severe disease. Mild influenza with non-specific symptoms is common, resulting in a large proportion of viral transmission and undetected infections.[6] In older children and adults, the illness characteristically begins abruptly with fever and a variety of clinical symptoms, including chills, malaise, headache, myalgia, non-productive cough, rhinitis, sore throat and mild conjunctivitis. Vomiting and diarrhoea may be present. While children aged under 5 years have fever, cough and rhinitis, infants may present with unexplained fever or sepsis-like syndrome only.[4] In the young, influenza virus may cause croup, bronchiolitis and pneumonia. Fever is often less evident in the elderly, who may present with other symptoms, such as anorexia, fatigue or confusion. Influenza typically resolves after several days in most people, although cough and malaise may persist for two or more weeks.
Infections due to pandemic influenza A strains are more likely to lead to severe morbidity and increased mortality than influenza B or seasonal influenza A strains.
Influenza B infections were previously thought to generally cause more mild illness, but numerous studies indicate that there is little difference between clinical symptoms and outcomes of influenza B compared to influenza A.[1] Influenza B-associated hospitalisations and mortality may have previously been underestimated; studies have reported higher mortality following influenza B infection than A in some years.[1] Influenza B infection is more common in children aged 5–17 years than in other age groups, and disease is likely to be more severe in children than in adults.[7]
Influenza can exacerbate underlying medical conditions, such as pulmonary, cardiac or metabolic disease. Some of the many reported complications associated with influenza include pneumonia, respiratory failure, myositis, encephalopathy, myocardial infarction, myocarditis and pericarditis, Reye syndrome (associated with aspirin use in children), bronchitis, otitis media and death. The risk of complications is increased in pregnancy.[8] Also associated with influenza infection is increased frailty and cognitive decline in older people and incidence of cardiovascular disease are also associated with influenza infection.[9, 10] Influenza during pregnancy can result in poorer outcomes for the mother and her fetus, including preterm birth and fetal loss.[11, 12]
Asymptomatic influenza
The majority of influenza infections are asymptomatic, and most symptomatic cases self-manage without seeking medical help.[13, 14] Results from the 2015 New Zealand Southern Hemisphere Influenza and Vaccine Effectiveness, Research and Surveillance (SHIVERS) serosurvey showed that around 32 percent of people surveyed had serologically confirmed influenza over the 2015 season (adjusted for age and ethnicity).[6, 15] Overall only one-quarter of those reported influenza-like illness; three out of four people were asymptomatic; only 1 out of 47 visited their GP and 1 in 680 were hospitalised. Young children and Pacific people experienced the highest influenza infection attack rates.[16]
11.3. Epidemiology
11.3.1. Global epidemiology
11.3.1. Global epidemiology
Influenza is an important cause of disease worldwide. Annual epidemics are estimated to result in about 3 to 5 million cases of severe illness, and about 290,000 to 650,000 respiratory deaths globally.[17, 18] For example, it was estimated globally that 11.5 percent of lower respiratory tract infections (LRTI), 5.6 percent of LRTI deaths and 9.5 million LRTI hospitalisations were attributable to influenza in 2017.[19]
In temperate climates, seasonal epidemics occur mainly during winter, while in tropical regions, influenza occurs throughout the year causing outbreaks more irregularly.[17]
From time to time, pandemics occur when a new virus arises and spreads globally (see section 11.3.3). The last influenza pandemic was caused by the A(H1N1)pdm09 virus. More than 214 countries and overseas territories reported laboratory-confirmed influenza, including over 18,449 deaths.[20] Many more deaths were found to associated with the pandemic due to respiratory and cardiovascular complications.[21]
11.3.2. New Zealand epidemiology
11.3.2. New Zealand epidemiology
New Zealand experiences the typical temperate climate epidemiology of influenza, with the peak incidence occurring during the winter months, however, influenza activity occurs throughout the year.
The impact of influenza in New Zealand is substantial in terms of general practice consultations, hospitalisations and deaths. The highest burden of disease is in the very young, the elderly, pregnant women, those with co-morbid conditions, people from low-income groups, and Pacific and Māori ethnic groups.
Influenza surveillance
The New Zealand influenza surveillance system compiles information from a variety of sources, including:
- national sentinel general practice-based influenza-like illness surveillance (part of the WHO’s Global Influenza Programme)
- year-round laboratory-based surveillance by the regional virus diagnostic laboratories
- hospital-based severe acute respiratory infection surveillance in Auckland and Counties Manukau districts
- data from Healthline, HealthStat, publicly funded hospital discharges, and the AIR.
Influenza prevalence and circulating strains are monitored through general practice surveillance for influenza-like illness (ILI); hospitalisations are monitored for severe acute respiratory infection (SARI) admissions; and severity is determined by the proportion of hospitalisations requiring intensive care unit (ICU) admission.[22]
Due to COVID-19 pandemic restrictions, transmission of influenza in the community was almost completely interrupted during 2020 and 2021.[23,24] However, a spike influenza occurred earlier than usual in 2022 with a high influenza-associated hospitalisation burden (see Figure 11.1).[25] Although the influenza activity was lower in 2023 than in 2022, the highest burden for influenza-associated SARI hospitalisations was seen in young children aged under 4 years and in older adults aged 65 and over. Likewise in 2024,[26] with a moderate peak in influenza-positive SARI hospitalisation seen during mid-July, the highest burden was in young children and older adults. By ethnic group, Pacific people, Māori and MELAA had higher rates of influenza-SARI hospitalisation than those of European, Asian and other ethnicities.[26]
Figure 11.1 Influenza-associated severe acute respiratory illness (SARI) hospitalisations in 2024, in comparison to pre-COVID-19 pandemic 2015-2019 and during pandemic 2020-2023. (source ESR, 2024)
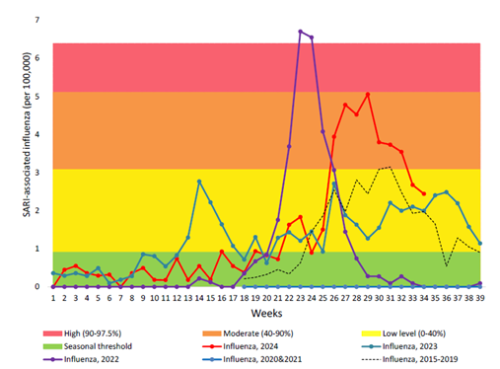
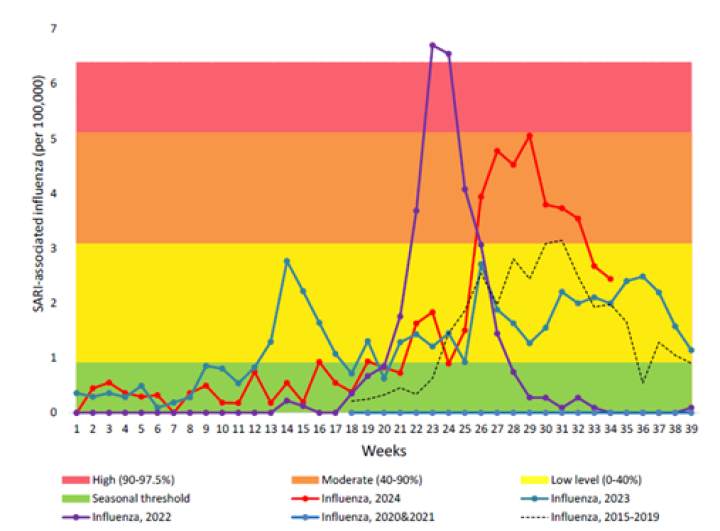
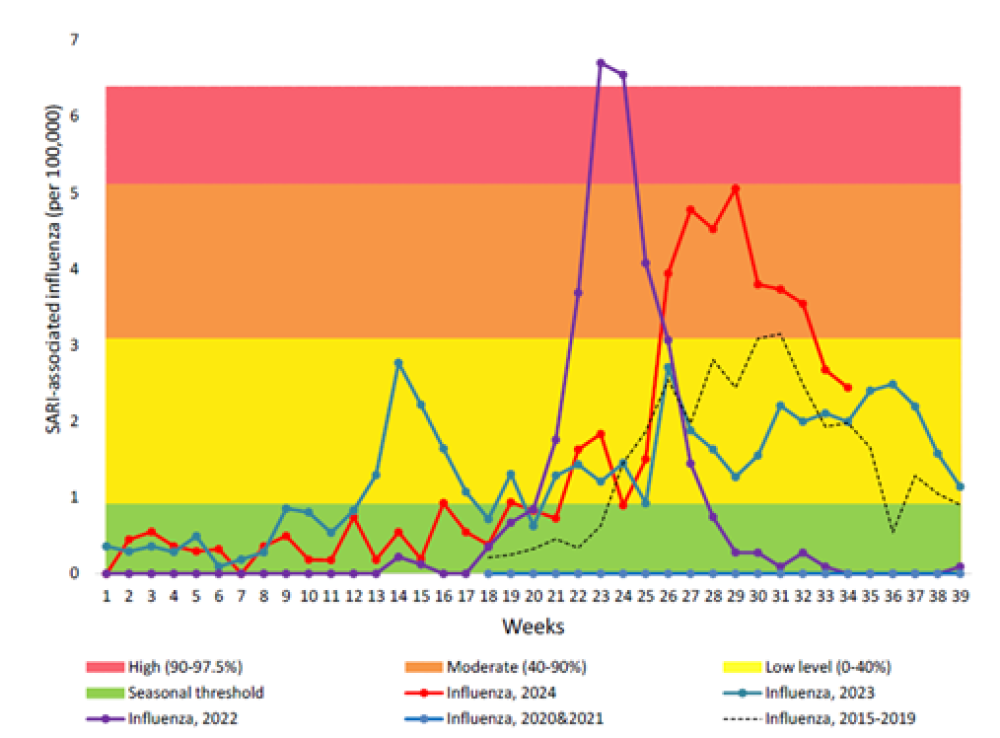
For detailed information on respiratory illness epidemiology, including influenza surveillance and influenza reports, see the PHF Science (formerly ESR) website (at https://www.phfscience.nz/digital-library/sti-quarterly-dashboard/ (external link) and https://www.phfscience.nz/digital-library/respiratory-illness-dashboard/ (external link)) (external link)
Influenza immunisation uptake
According to the Health New Zealand | Te Whatu Ora data (see website below), around 1.17 million influenza vaccinations were recorded in AIR between 2 April and 6 October 2024 (likely underestimate as some privately funded vaccinations may not have been recorded). This equated to just under one quarter of the whole population (approximately 224 doses per 1,000 population). According to AIR, the estimated influenza vaccine uptake for individuals aged 65 years and older was at least 61 percent in 2024. The uptake recorded in AIR for children aged 6 months to 12 years was only 5 percent, despite the high influenza burden in young children and high infection rates in all children.[26]
For details see https://www.tewhatuora.govt.nz/for-health-professionals/data-and-statistics/immunisation/national-influenza-vaccine-data (external link).
11.3.3. Pandemic influenza
11.3.3. Pandemic influenza
The natural ecology of influenza type A viruses is among wild aquatic avian species, and from time to time, these viruses spill over into other species, including humans. These avian influenza virus infections are usually severe and associated with a high mortality; however, they are rarely transmitted from human to human. In the past, avian viruses have become transmissible either through adaptation or the acquisition of swine or human genomic material, and when natural immunity has been lacking in the population, have resulted in a pandemic with global spread. There have been four influenza pandemics recorded since 1918.
Pandemics have the potential to result in large numbers of severe infections, but the degree of severity is hard to predict and will depend upon many factors, including whether there is any previous community immunity. The most severe recorded influenza pandemic was the ‘Spanish flu’ A(H1N1) pandemic of 1918–1920, which caused an estimated 20–50 million deaths worldwide. The most recent pandemic was the 2009 A(H1N1)pdm09 strain. It was estimated that 18 percent (800,000) of the New Zealand population were infected with the virus during the first wave, including one in every three children.[27] Risk factors for severe outcomes included obesity, pregnancy,[28] diabetes mellitus and Pacific or Māori ethnicity.[27] This strain is now established as a circulating seasonal influenza strain.
Globally, in the first 16 months of the 2009 H1N1 influenza pandemic, 18,500 deaths were attributed to laboratory-confirmed influenza. When investigated further, it was estimated that over 201,000 respiratory deaths and an additional 83,300 cardiovascular deaths were associated with the pandemic – producing a rate 15 times higher than the laboratory-confirmed deaths. Of these deaths, 80 percent were younger than 65 years of age.[21]
Monitoring, surveillance and response for new pandemic strains are in place, particularly with an increase in highly pathogenic avian influenza being detected in wild sea mammals and birds, and outbreaks in domestic poultry and cattle globally. See epidemiology in the Avian Influenza chapter of the Communicable Disease Control Manual (https://www.tewhatuora.govt.nz/for-health-professionals/clinical-guidance/communicable-disease-control-manual/avian-influenza (external link)).
11.4. Vaccines
Annual influenza vaccination is a most important measure for preventing influenza infection and mortality. New Zealand’s annual National Influenza Immunisation Programme campaign includes an annual influenza kit for health care professionals (available from the IMAC website (external link)) and a national education and communication programme.
11.4.1. Available vaccines
11.4.1. Available vaccines
Funded vaccines
One quadrivalent inactivated, surface antigen influenza vaccine is funded:
Influvac Tetra (Viatris) for adults and children aged 6 months and over
Each 0.5 mL dose contains 15 µg haemagglutinin for each of the four recommended influenza virus strains; other components and excipients include potassium chloride, monobasic potassium phosphate, dibasic sodium phosphate dihydrate, sodium chloride, calcium chloride dihydrate, magnesium chloride hexahydrate and water for injections to 0.5 mL. Trace amounts of the following may also be present in each 0.5 mL dose: ovalbumin (<0.1 µg), formaldehyde, cetrimonium bromide, sodium citrate, sucrose, gentamicin sulphate, tylosine tartrate, hydrocortisone and polysorbate 80.
Availability of other influenza vaccines, particularly the unfunded vaccines, can vary between and during the season depending on demand and supply. Further details on other available vaccines can be found at immune.org.nz (external link)
Vaccine preparations and potential future options
Influenza vaccine preparations vary by their type, the number of influenza strains contained in the vaccine and their delivery systems. There are a range of delivery mechanisms available internationally, including intradermal injection and intranasal mists. Live attenuated influenza vaccines are delivered by intranasal spray. Some data suggest that intradermal vaccines may induce improved immune responses, particularly in older adults.[29, 30]
The seasonal influenza vaccine strains vary each year depending on the prevailing viruses. WHO conducts technical consultations in February/March and September each year to recommend viruses for inclusion in both trivalent and quadrivalent vaccines for the northern and southern hemisphere influenza seasons, respectively. In recent years, the southern hemisphere recommendations include the two influenza type A (H1N1pdm09 and H3N2) and two B (Victoria and Yamagata) strains likely to circulate in New Zealand over the coming influenza seasons.[31]
Split virion influenza vaccines
Quadrivalent influenza vaccines (QIVs) contain two type A and two type B influenza strains. Some are split virion vaccines prepared from virus grown in embryonated hens’ eggs. The virus is purified, disrupted and inactivated by splitting with beta‑propiolactone or formaldehyde. QIVs offer broader protection against co‑circulating B‑strains and better effectiveness in seasons of B-strain mismatch than trivalent influenza vaccines (TIVs), which contain two influenza type A strains and one type B strain.[1]
Cell-based influenza vaccine
For the 2025 influenza season, a cell-based QIV (QIVc; Flucelvax, Seqirus) is available (unfunded) to those aged 6 months and over.
Egg-based and cell-based influenza vaccines differ in their manufacturing processes. Traditional egg-based vaccines involve cultivating influenza viruses in embryonated chicken eggs, with harvested viral particles subsequently purified and inactivated for vaccine production. In contrast, the cell-based influenza vaccine uses mammalian cell cultures to rapidly propagate large volumes of the influenza virus, eliminating the need for chicken eggs and enabling more rapid manufacturing. Cell-based influenza vaccine manufacture offers advantages in situations where egg-based manufacture faces challenges, such as egg shortages and egg adaptation, or when there is antigenic mismatch due to mutations in the circulating season influenza virus.
Some studies comparing the relative efficacy of egg-based and cell-based influenza vaccines have shown that a cell-based vaccine may have an advantage during certain seasons when the variations between the egg-based vaccine strains and the influenza strains circulating in the population are substantial.[32,33]
Adjuvanted vaccines
Adjuvants enhance the immune response to an antigen and enable the use of less antigen (antigen sparing). Internationally, there are three adjuvants licensed for use in influenza vaccines: two oil-in-water emulsions and a third that uses immune-potentiating reconstituted influenza virosomes.[4] Vaccines with these adjuvants show modestly improved immune responses, which may be particularly useful for the elderly and young children, but may also cause more local and systemic reactions than unadjuvanted vaccines.[4, 34]
For the 2025 influenza season, an adjuvanted QIV (aQIV; Fluad Quad, Seqirus) is available (unfunded) to those aged 65 years and over. It contains a squalene-based oil-in-water emulsion adjuvant (designated MF-59). This adjuvant has been used in influenza vaccines since 1997 and has a good safety record. Since there are limited head-to-head studies, a systematic review, comparing vaccine effectiveness of adjuvanted vaccine with the vaccine effectiveness of standard TIV or QIV or no vaccination found that adjuvanted TIV (aTIV) modestly improved vaccine effectiveness in adults aged 65 years or older.[35] Another systematic review of randomised clinical trials found no differences between adjuvanted vaccine and standard egg-based influenza vaccine.[36] However, when the same review analysed observational studies, adjuvanted vaccine were shown to modestly increase protection by 10 percent (95% CI 6 – 15 percent) against influenza-associated hospitalisation in adults aged 65 years and over when compared with standard influenza vaccines.[36] A benefit of adjuvanted vaccine in older adults was seen against hospitalisation for pneumonia, cerebrovascular and cardiovascular events over 15 influenza seasons in Italy when compared with standard non-adjuvanted vaccine (39 percent reduction, 95% CI 4-61 percent).[37]
Other influenza vaccines, not available in New Zealand
High dose vaccines
High-dose influenza vaccine formulations are not available in New Zealand. High-dose influenza vaccines containing four times more haemagglutinin antigen than standard vaccines have been shown to be more effective against influenza-related death and all-cause hospitalisation in the elderly than standard-dose trivalent vaccines.[38] A systematic review and meta-analysis of observational studies found that enhanced influenza vaccines (high-dose, adjuvanted and recombinant) were 11–18 percent more protective against influenza-hospitalisation of older adults compared with standard vaccine, and that high-dose vaccine had similar effectiveness to adjuvanted vaccine.[36]
Live attenuated influenza vaccines
At the time of writing, no live attenuated influenza vaccine (LAIV) is approved for use in New Zealand. LAIV (Fluenz, AstraZeneca, also known as FluMist in the US) is used routinely in all preschool and school-aged children (ages 2-17 years) in the UK. It has shown good effectiveness in children aged 6 months to 7[39] and other vaccinated cohorts.[40] Up to 60 percent protection against influenza-associated hospitalisation was shown in children aged 2–6 years over three influenza seasons in England.[41]
LAIV contains cold-adapted live attenuated influenza virus that replicates in the cooler nasal tissue and not the lower airways. By mimicking natural influenza infection and evoking both mucosal and systemic immunity, it can induce stronger immune responses and induce broader cellular immune responses than inactivated influenza vaccines administered intramuscularly, particularly in children.[42] As with other seasonal influenza vaccines, effectiveness can vary between seasons depending on match with circulating strains. Trivalent and quadrivalent LAIVs are licensed for use in North America for healthy non pregnant individuals aged 2–49 years and in Europe for children aged 2–18 years.[43]
11.4.2. Efficacy and effectiveness
11.4.2. Efficacy and effectiveness
International data
The efficacy (prevention of illness among vaccinated individuals in controlled trials) and effectiveness (prevention of illness in vaccinated populations) of influenza vaccine depends on several factors. The age and immune competence of the vaccine recipient are important factors, as well as the match between the virus strains in the vaccine and those in circulation each year. Mismatches can evolve during a season or due to mutations occurring during vaccine manufacture (egg adaptation).[44] Previous vaccination history has been suggested to reduce the vaccine effectiveness in some cases; possibly more so when the previous vaccination was mismatched with the circulating strains at the time.[45] More recently, prior-season vaccination history has not been associated with reduced vaccine effectiveness in children or adults, and findings support annual revaccination.[44, 46, 47, 48, 49] With increasing complexity, this continues to be researched.
Prior to 2020, two influenza B strains have frequently co-circulated, and due to the challenges involved in predicting which B strains will circulate in the upcoming season, mismatches between the B strain selected for TIVs and the circulating B strains have occurred in up to one-half of influenza seasons. The capacity of QIVs (containing two B influenza strains) to provide broader immune responses against B strains and cross-protection during B-mismatched seasons was expected to prevent more influenza cases, hospitalisations and deaths than TIVs.[1] The composition of future vaccines will consider the status of influenza B virology.
Data for vaccine efficacy and effectiveness of TIVs is summarised in Table 11.1.
Table 11.1: Current estimates of TIV influenza vaccine efficacy and effectiveness
|
Population |
Type of outcome |
Level of protection |
Ref |
|---|---|---|---|
|
Pregnant women |
Effectiveness
|
50% (15–71%) |
|
|
|
51 | |
|
Infants aged under 6 months whose mothers received an influenza vaccination during pregnancy |
Effectiveness
|
41% (7–63%) to |
|
|
47% (12–68%) |
53 | |
|
Healthy children
|
Effectiveness
|
Insufficient data under 2 years
|
|
|
66% (29–84%) |
55 | |
|
|
65% (47–78%) |
56 |
|
Efficacy against confirmed influenza |
64% (52–72%) |
54 |
|
Effectiveness
|
28% (21–35%) to |
|
|
|
56% (12–78%) |
57 | |
|
Children with high-risk conditions aged 6 months to 17 years |
Effectiveness against influenza-related death |
51% (31–67%) |
56 |
|
Healthy adults |
Effectiveness
|
59% (53–64%) to |
|
|
16% (5–25%) to 18% (2–31%) |
||
|
61% (34–77%) |
59 | |
|
55% (24–73%) |
||
|
Adults with high-risk conditions: |
|
|
|
|
|
17% reduced risk |
60 |
|
|
56% reduced risk |
61 |
|
Effectiveness against influenza-related hospitalisation |
22% (15–27%) to |
62 |
|
Adults aged 40 years or older |
Effectiveness against acute myocardial infarction |
29% (9%–44%) |
63 |
|
Adults aged 65 years or older |
Effectiveness
|
49% (33–62%) |
|
|
39% (35–43%) |
||
|
28% (26–30%) |
65 |
Vaccine effectiveness in New Zealand
New Zealand data is consistent with international data. While there is some variability from year to year and with different strains, the data overall show that the point estimate for influenza vaccine effectiveness is approximately 50 percent for preventing general practice visits, hospitalisations and for both influenza type A and B strains.[59, 66, 67, 68] Estimates for vaccine effectiveness tend to be higher in children and healthy midlife adults, and lower in the elderly. Influenza vaccination significantly reduces influenza-associated ICU admissions and attenuates disease severity in adults who were infected despite vaccination.[69]
The low influenza activity over recent years in New Zealand can cause imprecision in estimating annual vaccine effectiveness.[70,71] For the 2024 influenza season across all age groups, the estimated crude vaccine effectiveness against influenza-confirmed acute respiratory infection was 61 percent (95 percent CI: 48.6 – 70.4 percent) in the community (WellKiwis participants) For influenza-confirmed severe acute respiratory infection cases, the estimated crude vaccine effectiveness was 17.4 percent (95 percent CI: 0.6 to 31.3) in hospitalised patients. These were lower than reported in 2023.[26]
Pregnant women, the fetus and neonates
A pregnant woman and her fetus are at increased risk of influenza complications.[8] Physiological and immunological changes in pregnancy increase susceptibility to influenza.[72] Hospitalisation from influenza-related cardiorespiratory disorders during the second and third trimesters was especially apparent in the 2009 pandemic.[73] Influenza immunisation is therefore recommended during every pregnancy to reduce this risk, with similar effectiveness in healthy pregnant women as in other healthy adults against laboratory-confirmed influenza.[74] During the 2012–2013 seasons in Australia, women vaccinated in pregnancy were 81 percent less likely to attend emergency departments and 65 percent less likely to be hospitalised with acute respiratory illness than those unvaccinated.[51]
Influenza immunisation during pregnancy may reduce the incidence of stillbirth. Stillbirth was half as likely among vaccinated mothers compared to unvaccinated mothers in an Australian study.[12]
Maternal influenza immunisation offers protection to the newborn through maternal antibody transfer.[42, 53, 73, 75] Influenza vaccines are not registered and have not been shown to be effective in infants aged under 6 months: therefore, immunisation during pregnancy confers protection to newborns and infants who are too young to be vaccinated.[11, 52] Maternal influenza immunisation is significantly associated with reduced risk of influenza virus infection and influenza-related hospitalisation in infants up to 6 months of age and increased influenza antibody titres are maintained in infants through to age 2–3 months.[52, 76]
Children
Influenza vaccination in children provides similar protection to that seen in healthy adults. Influenza infection rates are generally highest in children. Effectiveness against laboratory-confirmed influenza is around 65 percent in young children aged 6 months to 5 years when vaccine and circulating strains are well‑matched.[54, 55, 56] Influenza vaccination offers the greatest protection against influenza-related hospitalisation to children who are fully immunised with routine vaccines.[77] QIV vaccine effectiveness against influenza hospitalisation of children in the 2018 season in Australia was estimated to be 78.8 percent (95% CI 66.9–86.4); this was when Australia expanded the funded influenza vaccination programme to preschool children, those with comorbid medical conditions and all indigenous children.[78]
The additional benefit of vaccinating children is protection of those around them, including their peers, grandparents and infants.
Healthy adults
Generally, randomised placebo-controlled trials of TIV in healthy adults support good protection against laboratory-confirmed influenza.[58] Effectiveness against laboratory-confirmed influenza is around 60 percent in adults, but varies with the match of vaccine with circulating strains (see Table 11.1).
Although currently available standard influenza vaccines are less effective at preventing clinical illness in older people,[79] influenza vaccination does reduce hospitalisation and deaths.
Effectiveness in community-dwelling adults aged over 60 years depends on how well the vaccine matches the circulating strains.[4] Influenza vaccine was moderately effective against laboratory-confirmed influenza during an epidemic season in community-dwelling adults age 65 year or older, irrespective of vaccine strain match. Significance was less during non-epidemic seasons and varied with virus type (the highest effectiveness was against A[H1N1] and the lowest against B).[80]
Vaccination has been demonstrated to prevent hospitalisation and death in older nursing home residents.[81, 82, 83, 84] A meta-analysis across 11 studies estimated influenza vaccination effectiveness to 37 percent (95% CI: 18–53; p=0.001) against pneumonia and 34 percent (95% CI: 10–53; p=0.01) against death due to pneumonia or influenza in institutionalised older adults.[85] A 2010 Cochrane review concluded that there was insufficient evidence to support influenza vaccine effectiveness in the elderly;[86] however, reanalysis of that review and its methodology argued that there is substantial evidence for the ability of influenza vaccine to reduce the risk of influenza infection and influenza-related disease and death in the elderly.[54, 64]
Severity of influenza symptoms are modestly attenuated by influenza vaccination in the elderly.[87] Therefore, by reducing severity of disease, vaccination can reduce the risk or duration of hospitalisation. Hospitalisation and immobility in the elderly leads to physical and mental decline, increased frailty and loss of independence.[9, 87, 88, 89]
Co-morbid conditions in adults and children
Influenza vaccination has been associated with reductions in hospitalisations and deaths among adults with risk factors for influenza complications, including diabetes,[90, 91] chronic obstructive pulmonary disease[92, 93] and heart failure.[60] Obese adults have a similar risk of influenza-associated hospitalisations as those with cardiovascular disease and diabetes.[94] Among Danish adults aged under 65 years with underlying medical conditions, vaccination reduced all-cause deaths by 78 percent and hospitalisations attributable to respiratory infections or cardiopulmonary diseases by 87 percent.[95] An Australian study of adults aged 40 years and older showed that unvaccinated adults are almost twice as likely as vaccinated adults to have an acute myocardial infarct.[96, 97]
Influenza vaccination is as effective as other preventative coronary care therapies (eg, smoking cessation, statins and antihypertensives) in protection against cardiovascular events.[97]
During the 2018 season in Australia, QIV vaccine effectiveness against influenza-related hospitalisation for children with comorbidities was estimated to be 77.3 percent (95% CI 59.8–87.2%).[78]
This highlights the importance of vaccinating children, as well as adults, with comorbidities against influenza.
Herd immunity
Influenza vaccination can provide indirect protection to those who are unimmunised or respond less well to the vaccine. This has been shown in certain settings, such as within schools and nursing homes.[4] There is evidence to suggest that herd immunity can be achieved, particularly by vaccinating children.[98]
The UK has progressively rolled-out a vaccination programme using LAIV and QIV, starting with children aged 2–3 years in 2013/14 and extended to children aged 4–7 years in 2015/16. As of the 2019/2020 season, influenza vaccine is offered in the UK to all children aged 2–10 years and up to 18 years for high risk groups. Early results from school-based pilot studies provided evidence of direct effect, indirect effects and overall impact, with decreases in disease incidence and influenza positivity in vaccinated and non-vaccinated groups.[99] A systematic review found that vaccination of children conferred indirect protection in some but not all settings.[100]
Some studies suggest that herd immunity may also be achieved in nursing homes if immunisation coverage of residents is greater than 80 percent.[101] Vaccinating health care workers is likely to be an effective strategy, particularly when in contact with high‑risk patients.[14]
As shown by New Zealand SHIVERS data,[6] most people who catch the virus are asymptomatic or have very mild symptoms but are at risk of spreading it, such that increased vaccine uptake (funded and unfunded) across the whole population, from 6 months of age, is likely to achieve the greatest protection.
Duration of immunity
Due to the continual drift of influenza viruses, duration of immunity provided by influenza vaccines is difficult to study. However, when the strains stay the same for consecutive years, vaccination in a previous year appears to confer immunity into the next year for healthy adults and children.[4, 43] However, shorter duration of immunity is likely in other groups, particularly the elderly.[43]
Protection due to LAIVs has been demonstrated to persist beyond a year.[102, 103]
11.4.3. Transport, storage and handling
11.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
Store in the dark at +2°C to +8°C. Do not freeze.
11.4.4. Dosage and administration
11.4.4. Dosage and administration
The funded quadrivalent influenza vaccine should be administered by intramuscular, or subcutaneous injection, if indicated (see section 2.2.3). The contents of the syringe must be shaken thoroughly before use.
Individuals aged 6 months and older
Individuals aged 6 months and older receive a single 0.5 mL intramuscular dose of a QIV vaccine.
Children aged under 9 years who have not previously received influenza vaccine require two doses of vaccine four weeks apart to produce a satisfactory immune response.
Immunocompromised individuals
Regardless of their age, previously unvaccinated immunocompromised individuals or those who have received a solid organ or haematopoietic stem cell transplant are recommended to receive two doses of influenza vaccine, four weeks apart (the second dose is unfunded).[104] One dose is then given in each subsequent year. (See section 4.2.5.)
Co-administration with other vaccines
Influenza vaccine can be coadministered with other vaccines, such as COVID-19 vaccines (mRNA-CV), pneumococcal polysaccharide vaccine (23PPV), tetanus diphtheria acellular pertussis (Tdap) vaccine, and all the scheduled childhood vaccines, given at separate injection sites. Concurrent administration of influenza vaccine and 13-valent pneumococcal conjugate vaccine (PCV13) carries an increased risk of fever.[105, 106] Separation of the vaccines by two days can be offered, but is not essential. (See also section 17.6.2.)
11.5. Recommended immunisation schedule
The optimal time to vaccinate people against influenza, particularly those in high-risk groups, is generally recommended from 1 April, annually, in advance of the usual May to September period of influenza virus activity. The vaccine can be given even when influenza virus activity has been identified, because protective antibody levels develop from four days after immunisation, with full protection after two weeks.[107] The vaccine should be administered annually to maintain immunity and to provide protection against new strains.
Vaccine effectiveness may be reduced in those at highest risk from influenza. Influenza vaccine is therefore recommended annually for everyone from the age of 6 months to reduce the spread of influenza virus and to protect against influenza-related complications. It is particularly important to vaccinate contacts of high-risk individuals, such as family and caregivers, and those working in certain occupations. See Table 11.2 for a summary of the funded and unfunded recommendations for influenza immunisation.
See the national 2024 Influenza Immunisation Programme Campaign website (external link) for further information.
Table 11.2: Influenza vaccine recommendations
Table 11.2: Influenza vaccine recommendations
| Note: Funded individuals are in the shaded rows. Refer to the Pharmaceutical Schedule (external link) for any changes to the funding decisions. |
|
Recommended and funded |
|---|
|
All individuals aged 65 years and older. |
|
Individuals aged 6 months to under 65 years who:
|
|
Recommended but not funded |
|
Generally, this vaccine is recommended annually for all individuals age from 6 months; it is particularly important for:
|
11.5.1. Pregnancy and breastfeeding
11.5.1. Pregnancy and breastfeeding
The influenza vaccine is strongly recommended, and funded, for people who will be pregnant while the vaccine is available. Pregnant women can receive influenza vaccination at any stage of pregnancy to protect themselves, their fetus and their newborn for each season they are pregnant. When pregnancy spans two influenza seasons, two vaccinations (one from each season) are recommended to protect against all the predicted strains.
Influenza vaccine is safe to administer during any stage of pregnancy or while lactating. There is no evidence that influenza vaccine prepared from inactivated split virus or subunits causes damage to the fetus or neonate[108] and there is some evidence it may be protective against stillbirth.[12]
Pregnant women are at greater risk from complications associated with influenza illness.[8, 11] When pregnancy is superimposed on high-risk conditions such as asthma or diabetes, influenza-related morbidity is three to four times greater than in non‑pregnant women with similar high-risk conditions.
Globally, about one-quarter of influenza-associated hospital admissions and over one-third of in-hospital deaths are in infants under 6 months.[109] Because there is no registered or effective vaccine for children aged under 6 months, vaccination during pregnancy is highly recommended to improve maternal-fetal passive antibody transfer.[11] Influenza vaccination of pregnant women has been shown to significantly decrease influenza in their newborn babies.[42, 53, 73, 75] Breastfeeding is also recommended, to deliver passive immunity to the infant.[42] (See also section 4.1.2.)
11.5.2. Children at increased risk
11.5.2. Children at increased risk
Influenza vaccine is recommended and funded for children aged 6 months and over with certain chronic illnesses (see Table 11.2) and for children aged under 4 years a history of significant respiratory disease (including a history of measles). Children with the following conditions should be prioritised to receive influenza vaccine due to their increased risk, including:
- all asthmatics on regular preventive therapy
- other children with chronic respiratory disorders (eg, cystic fibrosis, non-cystic fibrosis bronchiectasis and chronic lung disease of infancy).
Special considerations apply to children, as follows (see also section 4.2.5):
- Immunisation is occasionally associated with fever between 6 and 24 hours after administration. In children aged 6–24 months with significant chronic medical conditions fever may cause an exacerbation of the underlying condition.
- Children receiving cancer chemotherapy may have a weaker response to influenza vaccine. Vaccination is recommended three to four weeks after the preceding dose of chemotherapy, when the neutrophil and lymphocyte counts are each ≥1.0 × 109/L. Children who are no longer receiving chemotherapy can be expected to show seroconversion to vaccine three months after the cessation of chemotherapy.
11.5.3. Adults at increased risk
11.5.3. Adults at increased risk
Adults aged 65 years and older
In adults aged 65 years and older, influenza vaccine has been shown to be effective against non-fatal and fatal influenza complications, influenza-like illness and laboratory-confirmed influenza (see Table 11.1). Influenza vaccination protects against loss of independence due to increasing levels of frailty associated with hospitalisation.[9, 89, 110]
Adults with underlying medical conditions
Influenza has been associated with increased morbidity and mortality in adults with underlying medical conditions (see Table 11.2). Risk increases with multiple conditions. These also include those with serious mental health conditions and accessing mental health or addiction services. (See also Pregnancy and breastfeeding).
11.5.4. Recommended but not funded
11.5.4. Recommended but not funded
Generally, influenza vaccination is recommended annually for all individuals aged from 6 months. It is particularly important for the groups listed in Table 11.2.
There are certain conditions that individually do not render a person eligible for funded influenza vaccine, but when combined, significantly increase the risk of influenza complications (this is described as ‘risk stacking’). Such risks are further increased by smoking, alcohol dependency and obesity.
To optimise the protection of high-risk infants and toddlers, including those aged under 6 months, all household members and close contacts are recommended to receive influenza vaccine (not funded between ages 13–64 years, unless eligibility criteria are met). Influenza vaccine is recommended and funded to be received at any stage of pregnancy, for those aged 65 years or over and for children aged 6 months to 12 years.
Adults of Māori or Pacific ethnicity aged 55 years and over
Māori and Pacific people are at greater risk of developing underlying health conditions, such as cardiovascular disease and chronic respiratory disease, at a younger age than other ethnicities,[111] which increases the risk of influenza severity and complications. Factors contributing to this risk an increased risk of respiratory virus transmission can included overcrowding due to lack of appropriate multigenerational housing and close-knit communities.
Healthy individuals of any age from 6 months and older
Healthy individuals are encouraged to have an influenza vaccine, annually, especially if they are in close contact with individuals at high risk of complications. Employers are encouraged to provide influenza vaccine to avoid illness in their employees, especially those engaged in health care and other essential community services (see Table 4.9). Immunising healthy individuals has been shown to be cost-effective.
To optimise the protection of high-risk infants and toddlers, including those aged under 6 months (see Table 11.2), all household members and close contacts are recommended to receive influenza vaccine (not funded for those aged under 65 years, unless eligibility criteria are met). Influenza vaccine is recommended and funded to be received at any stage of pregnancy, for those aged 65 years or over.
Health care workers
Health New Zealand | Te Whatu Ora strongly recommends, and expects, that all health care workers will receive annual influenza vaccination for their own protection and the protection of those in their care.
Travellers
Influenza vaccine is recommended for people travelling outside New Zealand, especially those who are in the at-risk groups who have not received vaccine during the previous autumn, depending on the season and their destination. In tropical countries, influenza activity can occur throughout the year but is more likely during the winter (wet) and summer seasons, while in the northern hemisphere activity is commonest between the months of December and March. Outbreaks of influenza among organised tourist groups (eg, on cruise ships) can occur throughout the year.
11.6. Contraindications and precautions
See also section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 for general contraindications for all vaccines.
11.6.1. Contraindications
11.6.1. Contraindications
Influenza vaccine should not be administered to people with a history of an anaphylactic reaction to a prior dose of influenza vaccine or to a vaccine component. Egg allergy, including anaphylaxis, is not a contraindication or precaution: see section 11.6.3.
11.6.2. Precautions
11.6.2. Precautions
History of Guillain–Barré syndrome
Influenza vaccination has been suggested to increase the risk of GBS. However, no association was found between administering 16 million doses of influenza vaccine and GBS in adults aged from 65 years in the US.[112] Any potential risk increase would be less than one additional case per million doses administered.[4, 43, 113] The risk of developing GBS is increased following influenza infection, and the magnitude of the risk is several times greater than that possibly occurring following influenza vaccination.[4, 113, 114]
New Zealand hospitalisations for GBS showed no increase during the 1990s despite the marked increase in vaccine use during this period but apparent year-to-year variation was observed. In particular, the doubling of vaccine use (with the introduction of funded vaccine) in 1997 was not associated with any increase in GBS hospitalisations. No excess risk for GBS following influenza vaccine in children has been documented. No association between influenza vaccines and any other neurological disease has been substantiated.
The risks and benefits of withholding vaccination should be considered on an individual basis, based on the potential morbidity and mortality associated with influenza for that individual, including the potential risk of recurrent GBS following influenza infection.
Co-administration with PCV13
Individuals (or their parents/guardians) who receive both influenza vaccine and 13‑valent pneumococcal conjugate vaccine (PCV13) should be advised of the increased risk of fever following concomitant administration of these vaccines.[105, 106, 115] Separation of the vaccines by two days can be offered, but is not essential (see also section 17.6.2).
11.6.3. Egg allergy
11.6.3. Egg allergy
Influenza vaccine can be safely administered to people with a history of egg allergy, including anaphylaxis, at general practices, pharmacies or at the workplace.[116]
Reported cases of anaphylaxis after influenza vaccination in egg-allergic individuals all occurred over 30 years ago, at a time when vaccine egg (ovalbumin) content was much higher than it is now. Recent studies have shown that influenza vaccines containing less than 1 µg of ovalbumin do not trigger anaphylaxis in sensitive individuals.[116]
11.7. Potential responses and AEFIs
Split virion or subunit influenza vaccines are generally well tolerated. The safety profile of quadrivalent vaccines is comparable to that of trivalent vaccines.[43]
Potential responses associated with these influenza vaccines in adults and children include pain, redness and/or swelling at the site of injection (10–64 percent of recipients, lasting less than two days).[4] These local inflammatory responses are almost always mild. Systemic events such as headache, muscle aches and fatigue may occur in adults. Passive reporting of local and systemic reactions to influenza vaccines is more frequent for females (both young and older adults) than males.[117] Australian surveillance data (collected by AusVaxSafety) found that just over 6 percent of adults reported any adverse event following seasonal influenza vaccination, of which less than 1 percent were systemic responses (fever, rash and seizure).[118]
Systemic reactions are more likely in children not previously exposed to the vaccine or virus, these are generally self-limiting and resolve within one to two days.[43] A large post-licensure study in the US, which reviewed more than 250,000 children aged under 18 years given influenza vaccine, showed no increase in clinically important medically attended events for two weeks after vaccination compared to control periods.[119]
In early 2010, an increase in febrile seizures in children in both Australia and New Zealand were all linked to the Fluvax brand influenza vaccine. Active surveillance in Australia continues to monitor for potential safety signals.
Vaccinators need to emphasise to recipients that:
- the split virion vaccine contains components of the virus, not the intact virus, and cannot cause influenza
- local reaction and mild systemic symptoms may occur within a day or two of immunisation
- respiratory viral infections are common, and many individuals will develop one coincidentally following immunisation, and these should not be falsely attributed to the vaccine.
An association was found in 2010 between narcolepsy and one H1N1 pandemic vaccine (Pandemrix, an adjuvanted vaccine not licensed or used in New Zealand). Data from various European countries support a temporal link.[120, 121, 122] The onset of narcolepsy may be confounded by other factors, such as genetic predisposition, A(H1N1)pdm09 influenza and/or other environmental factors.[123, 124, 125] A 2018 systematic review found that although the risk of narcolepsy type 1 increased in association with this particular vaccine, it remains a rare disease and the benefit of the influenza vaccination outweighs the risk.[126]
11.8. Public health measures
11.8.1. Improving vaccine uptake
11.8.1. Improving vaccine uptake
Studies in New Zealand and overseas have found that provider attitudes and provider recommendations are key to improving influenza vaccine uptake. Organised registers for recall and opportunistic immunisation are also likely to be important factors in achieving high uptake.
Every effort should be made during April to immunise all people at risk, particularly those aged 65 years and older, those aged under 65 years (including children) who have certain medical conditions, pregnant women and health care workers, and those of Māori or Pacific ethnicity aged 55 to 64 years. During an influenza outbreak, recommend influenza vaccination to anyone at risk who was not immunised during the current season or to those who have not received an influenza vaccination for more than six months. Availability of an appropriate vaccine is the most pertinent of these factors.
Vaccination of all healthy adults and children from age 6 months is encouraged but not funded. Adult vaccination, especially for those in close contact with high-risk groups, may be funded by employers.
11.8.2. Antiviral drugs
11.8.2. Antiviral drugs
Influenza antiviral drugs can be used to treat or to prevent influenza and can be adjuncts to influenza vaccination. Early use of antivirals, especially within the first 48 hours of illness, can reduce the duration of symptoms and the risk of complications from influenza. They are likely to be most effective against severe influenza and for those with high-risk comorbidities. Antivirals should be particularly considered for unimmunised or recently immunised contacts who are at high risk of severe disease to lower disease severity and improve recovery time. [127, 128]
11.8.3. Influenza pandemics
11.8.3. Influenza pandemics
At the time of a pandemic, recommended public health advice, priority groups and the timing of vaccination may be quite different from those during inter-pandemic periods. The New Zealand Pandemic Plan: A framework for action (external link) describes the key phases of a pandemic and the actions and responsibilities within each phase.
11.9. Variations from the vaccine data sheet
None.
References
References
References
- Ray R, Dos Santos G, Buck PO, et al. A review of the value of quadrivalent influenza vaccines and their potential contribution to influenza control. Human Vaccines & Immunotherapeutics, 2017. 13(7): p. 1640-1652.
- Paget J, Caini S, Del Riccio M, et al. Has influenza B/Yamagata become extinct and what implications might this have for quadrivalent influenza vaccines? Euro Surveillance, 2022. 27(39).
- Fine P, Mulholland K, Scott J,Edmunds W. 2018. Community Protection, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, and Edwards K (eds). Elsevier: Philadelphia, US.
- Bresee JS. 2018. Inactivated influenza vaccines, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, and Edwards K (eds). Elsevier: Philadelphia, US.
- Lowen AC, Mubareka S, Steel J,Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathogens, 2007. 3(10): p. 1470-6.
- Huang QS ,(on behalf of the SHIVERS Investigation team), Key Findings – SHIVERS (updated January 2017), in Presented at the 2016 New Zealand Influenza Symposium. 2016: Wellington
- Caini S, Huang QS, Ciblak MA, et al. Epidemiological and virological characteristics of influenza B: results of the Global Influenza B Study. Influenza Other Respir Viruses, 2015. 9(Suppl 1): p. 3-12.
- Prasad N, Huang QS, Wood T, et al. Influenza-associated outcomes among pregnant, postpartum, and nonpregnant women of reproductive age. Journal of Infectious Diseases, 2019. 219(12): p. 1893-1903.
- Andrew MK, Shinde V, Ye L, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. Journal of Infectious Diseases, 2017. 216(4): p. 405-414.
- Warren-Gash C, Smeeth L ,Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infectious Diseases, 2009. 9(10): p. 601-10.
- Marshall H, McMillan M, Andrews RM, et al. Vaccines in pregnancy: The dual benefit for pregnant women and infants. Human Vaccines & Immunotherapeutics, 2016. 12(4): p. 848-56.
- Regan AK, Moore HC, de Klerk N, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clinical Infectious Diseases, 2016. 62(10): p. 1221-7.
- Fragaszy EB, Warren-Gash C, White PJ, et al. Effects of seasonal and pandemic influenza on health-related quality of life, work and school absence in England: Results from the Flu Watch cohort study. Influenza Other Respir Viruses, 2018. 12(1): p. 171-182.
- Hayward AC, Harling R, Wetten S, et al. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomised controlled trial. BMJ, 2006. 333(7581): p. 1241.
- Huang QS ,(on behalf of the SHIVERS Investigation team), Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance (SHIVERS) & beyond, in Presented at the 4th New Zealand Influenza Symposium. 2018: Wellington
- Huang QS, Bandaranayake D, Wood T, et al. Risk factors and attack rates of seasonal influenza infection: Results of the Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance (SHIVERS) Seroepidemiologic Cohort Study. Journal of Infectious Diseases, 2019. 219(3): p. 347-357.
- World Health Organization. 2018 Influenza (Seasonal). 2018 [updated 6 November 2018]; URL: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal). (accessed 8 November 2019)
- Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet, 2018. 391(10127): p. 1285-1300.
- GBD 2017 Influenza collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med, 2019. 7(1): p. 69-89.
- World Health Organization. 2010 Pandemic (H1N1) 2009 – Update 112 (6 August 2010). Weekly update Weekly update; 2010; URL: http://www.who.int/csr/don/2010_08_06/en/. (accessed 3 July 2020)
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infectious Diseases, 2012. 12(9): p. 687-95.
- Huang QS ,(on behalf of the SHIVERS Investigation team), Flu in NZ and SHIVERS II update, in Presented at the 5th New Zealand Influenza Symposium. 2019: Wellington
- Institute of Environmental Science and Research (ESR). 2020 2020 Annual Influenza Summary. Porirua. URL: https://surv.esr.cri.nz/PDF_surveillance/Virology/FluAnnRpt/InfluenzaAnn2020.pdf. (accessed 28 March 2022)
- Institute of Environmental Science and Research (ESR). 2021 2021 Annual Influenza Summary. Porirua. URL: https://surv.esr.cri.nz/PDF_surveillance/Virology/FluAnnRpt/InfluenzaAnn2021.pdf. (accessed 28 March 2022)
- Institute of Environmental Science and Research (ESR). 2023 2022 Acute respiratory illness surveillance report. URL: https://www.esr.cri.nz/digital-library/influenza-annual-report-2022/. (accessed 12 February 2024)
- WHO National Influenza Centre and Health Intelligence Team ,Institute of Environmental Science and Research. 2024 Recommendation for seasonal influenza vaccine composition for New Zealand for 2025. URL: https://www.esr.cri.nz/digital-library/influenza-vaccine-recommendations-report-for-2025/. (accessed 19 February 2025)
- Institute of Environmental Science and Research Ltd. 2009. Seroprevalence of the 2009 Influenza A (H1N1) Pandemic in New Zealand (ed.), Porirua: Institute of Environmental Science and Research Ltd. URL: https://www.health.govt.nz/publication/seroprevalence-2009-influenza-h1n1-pandemic-new-zealand (accessed 19 March 2020)
- Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA, 2010. 303(15): p. 1517-25.
- Marra F, Young F, Richardson K,Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respir Viruses, 2013. 7(4): p. 584-603.
- Patel SM, Atmar RL, El Sahly HM, et al. Direct comparison of an inactivated subvirion influenza A virus subtype H5N1 vaccine administered by the intradermal and intramuscular routes. Journal of Infectious Diseases, 2012. 206(7): p. 1069-77.
- World Health Organization. 2019 Recommended composition of influenza virus vaccines for use in the 2020 southern hemisphere influenza season. WHO; 2019 [updated 27 September 2019]; URL: https://www.who.int/influenza/vaccines/virus/recommendations/2020_south/en/. (accessed 8 November 2019)
- Boikos C, McGovern I, Molrine D, et al. Review of Analyses Estimating Relative Vaccine Effectiveness of Cell-Based Quadrivalent Influenza Vaccine in Three Consecutive US Influenza Seasons. Vaccines (Basel), 2022. 10(6).
- Coleman BL, Gutmanis I, McGovern I,Haag M. Effectiveness of Cell-Based Quadrivalent Seasonal Influenza Vaccine: A Systematic Review and Meta-Analysis. Vaccines (Basel), 2023. 11(10).
- Tregoning JS, Russell RF ,Kinnear E. Adjuvanted influenza vaccines. Human Vaccines & Immunotherapeutics, 2018. 14(3): p. 550-564.
- Coleman BL, Sanderson R, Haag MDM,McGovern I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir Viruses, 2021. 15(6): p. 813-823.
- Ferdinands JM, Blanton LH, Alyanak E, et al. Protection against influenza hospitalizations from enhanced influenza vaccines among older adults: A systematic review and network meta-analysis. Journal of the American Geriatrics Society, 2024. 72(12): p. 3875-3889.
- Lapi F, Marconi E, Simonetti M, et al. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Expert Rev Vaccines, 2019. 18(6): p. 663-670.
- National Advisory Committee on Immunization (NACI), An Advisory Committee Review National Advisory Committee on Immunization (NACI): Literature Review Update on the Efficacy and Effectiveness of High-Dose (Fluzone® High-Dose) and MF59-Adjuvanted (Fluad®) Trivalent Inactivated Influenza Vaccines in Adults 65 Years of Age and Older. 2017, Public Health Agency of Canada.
- Osterholm MT, Kelley NS, Sommer A,Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infectious Diseases, 2012. 12(1): p. 36-44.
- Yin JK, Heywood AE, Georgousakis M, et al. Systematic Review and Meta-analysis of Indirect Protection Afforded by Vaccinating Children Against Seasonal Influenza: Implications for Policy. Clinical Infectious Diseases, 2017. 65(5): p. 719-728.
- Boddington NL, Mangtani P, Zhao H, et al. Live-attenuated influenza vaccine effectiveness against hospitalization in children aged 2-6 years, the first three seasons of the childhood influenza vaccination program in England, 2013/14-2015/16. Influenza Other Respir Viruses, 2022. 16(5): p. 897-905.
- Esposito S, Tagliabue C, Tagliaferri L, et al. Preventing influenza in younger children. Clinical Microbiology and Infection, 2012. 18 Suppl 5(Suppl 5): p. 42-9.
- Grohskǿpf LA, Sokolow LZ, Broder KR, et al. 2016. Prevention and control of seasonal influenza with vaccines. MMWR: Recommendations and Reports. 65(5): p. 1-54. DOI: 10.15585/mmwr.rr6505a1 (accessed 9 March 2020)
- Pebody R, Djennad A, Ellis J, et al. End of season influenza vaccine effectiveness in adults and children in the United Kingdom in 2017/18. Euro Surveillance, 2019. 24(31).
- Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: Impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014-2015 season. Clinical Infectious Diseases, 2016. 63(1): p. 21-32.
- Bartoszko JJ, McNamara IF, Aras OAZ, et al. Does consecutive influenza vaccination reduce protection against influenza: A systematic review and meta-analysis. Vaccine, 2018. 36(24): p. 3434-3444.
- Cheng AC, Macartney KK, Waterer GW, et al. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clinical Infectious Diseases, 2017. 64(11): p. 1564-1572.
- McLean HQ, Caspard H, Griffin MR, et al. Association of prior vaccination with influenza vaccine effectiveness in children receiving live attenuated or inactivated vaccine. JAMA Netw Open, 2018. 1(6): p. e183742.
- Ramsay LC, Buchan SA, Stirling RG, et al. The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Medicine, 2017. 15(1): p. 159.
- Madhi SA, Cutland CL, Kuwanda L, et al. Influenza vaccination of pregnant women and protection of their infants. New England Journal of Medicine, 2014. 371(10): p. 918-31.
- Regan AK, Klerk N, Moore HC, et al. Effectiveness of seasonal trivalent influenza vaccination against hospital-attended acute respiratory infections in pregnant women: A retrospective cohort study. Vaccine, 2016. 34(32): p. 3649-56.
- Eick AA, Uyeki TM, Klimov A, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Archives of Pediatrics and Adolescent Medicine, 2011. 165(2): p. 104-11.
- Poehling KA, Szilagyi PG, Staat MA, et al. Impact of maternal immunization on influenza hospitalizations in infants. American Journal of Obstetrics and Gynecology, 2011. 204(6 Suppl 1): p. S141-8.
- Jefferson T, Rivetti A, Di Pietrantonj C,Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev, 2018. 2: p. CD004879.
- Heinonen S, Silvennoinen H, Lehtinen P, et al. Effectiveness of inactivated influenza vaccine in children aged 9 months to 3 years: an observational cohort study. The Lancet Infectious Diseases, 2011. 11(1): p. 23-9.
- Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics, 2017. 139(5).
- Blyth CC, Macartney KK, Hewagama S, et al. Influenza epidemiology, vaccine coverage and vaccine effectiveness in children admitted to sentinel Australian hospitals in 2014: the Influenza Complications Alert Network (FluCAN). Euro Surveillance, 2016. 21(30).
- Demicheli V, Jefferson T, Ferroni E, et al. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev, 2018. 2: p. CD001269.
- Turner N, Pierse N, Bissielo A, et al. Effectiveness of seasonal trivalent inactivated influenza vaccine in preventing influenza hospitalisations and primary care visits in Auckland, New Zealand, in 2013. Euro Surveillance, 2014. 19(34).
- Rodrigues BS, David C, Costa J, et al. Influenza vaccination in patients with heart failure: a systematic review and meta-analysis of observational studies. Heart, 2019.
- Wang IK, Lin CL, Chang YC, et al. Effectiveness of influenza vaccination in elderly diabetic patients: a retrospective cohort study. Vaccine, 2013. 31(4): p. 718-24.
- Gershon AS, Chung H, Porter J, et al. Influenza vaccine effectiveness in preventing hospitalizations in older patients with chronic obstructive pulmonary disease. Journal of Infectious Diseases, 2019.
- Barnes M, Heywood AE, Mahimbo A, et al. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart, 2015. 101(21): p. 1738-47.
- Beyer WE, McElhaney J, Smith DJ, et al. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine, 2013. 31(50): p. 6030-3.
- Demicheli V, Jefferson T, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev, 2018. 2: p. CD004876.
- Bissielo A, Pierse N, Huang QS, et al. Effectiveness of seasonal influenza vaccine in preventing influenza primary care visits and hospitalisation in Auckland, New Zealand in 2015: interim estimates. Euro Surveillance, 2016. 21(1).
- Pierse N, Kelly H, Thompson MG, et al. Influenza vaccine effectiveness for hospital and community patients using control groups with and without non-influenza respiratory viruses detected, Auckland, New Zealand 2014. Vaccine, 2016. 34(4): p. 503-509.
- Turner N, Pierse N, Bissielo A, et al. The effectiveness of seasonal trivalent inactivated influenza vaccine in preventing laboratory confirmed influenza hospitalisations in Auckland, New Zealand in 2012. Vaccine, 2014. 32(29): p. 3687-93.
- Thompson MG, Pierse N, Sue Huang Q, et al. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012-2015. Vaccine, 2018. 36(39): p. 5916-5925.
- Institute of Environmental Science and Research (ESR). 2018 2018 Annual Influenza Summary. ESR; 2018; URL: https://surv.esr.cri.nz/virology/influenza_annual_report.php. (accessed 3 July 2020)
- Huang QS, Jelley L, Bocacao J, et al., Recommendations for Season Influenza Vaccine Composition for New Zealand 2019. 2018, Institute of Environmental Science and Research: Wellington.
- Sakala IG, Honda-Okubo Y, Fung J,Petrovsky N. Influenza immunization during pregnancy: Benefits for mother and infant. Human Vaccines & Immunotherapeutics, 2016. 12(12): p. 3065-3071.
- Tamma PD, Ault KA, del Rio C, et al. Safety of influenza vaccination during pregnancy. American Journal of Obstetrics and Gynecology, 2009. 201(6): p. 547-52.
- Quach THT, Mallis NA ,Cordero JF. Influenza vaccine efficacy and effectiveness in pregnant women: systematic review and meta-analysis. Matern Child Health J, 2020. 24(2): p. 229-240.
- Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. New England Journal of Medicine, 2008. 359(15): p. 1555-64.
- Dabrera G, Zhao H, Andrews N, et al. Effectiveness of seasonal influenza vaccination during pregnancy in preventing influenza infection in infants, England, 2013/14. Euro Surveillance: Bulletin Europeen sur les Maladies Transmissibles = European Communicable Disease Bulletin, 2014. 19(45): p. 20959.
- Kalligeros M, Shehadeh F, Mylona EK, et al. Influenza vaccine effectiveness against influenza-associated hospitalization in children: A systematic review and meta-analysis. Vaccine, 2020. 38(14): p. 2893-2903.
- Blyth CC, Cheng AC, Crawford NW, et al. The impact of new universal child influenza programs in Australia: Vaccine coverage, effectiveness and disease epidemiology in hospitalised children in 2018. Vaccine, 2020. 38(13): p. 2779-2787.
- Govaert TM, Thijs CT, Masurel N, et al. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA, 1994. 272(21): p. 1661-5.
- Darvishian M, van den Heuvel ER, Bissielo A, et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: an individual participant data meta-analysis of test-negative design case-control studies. Lancet Respir Med, 2017. 5(3): p. 200-211.
- Deguchi Y, Takasugi Y ,Tatara K. Efficacy of influenza vaccine in the elderly in welfare nursing homes: reduction in risks of mortality and morbidity during an influenza A (H3N2) epidemic. Journal of Medical Microbiology, 2000. 49(6): p. 553-6.
- Gross PA, Hermogenes AW, Sacks HS, et al. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Annals of Internal Medicine, 1995. 123(7): p. 518-27.
- Gross PA, Quinnan GV, Rodstein M, et al. Association of influenza immunization with reduction in mortality in an elderly population. A prospective study. Archives of Internal Medicine, 1988. 148(3): p. 562-5.
- Saah AJ, Neufeld R, Rodstein M, et al. Influenza vaccine and pneumonia mortality in a nursing home population. Archives of Internal Medicine, 1986. 146(12): p. 2353-7.
- Chan TC, Fan-Ngai Hung I, Ka-Hay Luk J, et al. Effectiveness of influenza vaccination in institutionalized older adults: a systematic review. Journal of the American Medical Directors Association, 2014. 15(3): p. 226 e1-226 e6.
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev, 2010(2): p. CD004876.
- Mosnier A, Daviaud I, Caini S, et al. Does seasonal vaccination affect the clinical presentation of influenza among the elderly? A cross-sectional analysis in the outpatient setting in France, 2003-2014. Vaccine, 2017. 35(16): p. 2076-2083.
- Brummel NE, Balas MC, Morandi A, et al. Understanding and reducing disability in older adults following critical illness. Critical Care Medicine, 2015. 43(6): p. 1265-75.
- Gill TM, Allore HG, Holford TR,Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA, 2004. 292(17): p. 2115-24.
- Looijmans-Van den Akker I, Verheij TJ, Buskens E, et al. Clinical effectiveness of first and repeat influenza vaccination in adult and elderly diabetic patients. Diabetes Care, 2006. 29(8): p. 1771-6.
- Vamos EP, Pape UJ, Curcin V, et al. Effectiveness of the influenza vaccine in preventing admission to hospital and death in people with type 2 diabetes. CMAJ: Canadian Medical Association Journal, 2016. 188(14): p. E342-E351.
- Poole PJ, Chacko E, Wood-Baker RW,Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev, 2006(1): p. CD002733.
- Kopsaftis Z, Wood-Baker R ,Poole P. Influenza vaccine for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev, 2018. 6: p. CD002733.
- Karki S, Muscatello DJ, Banks E, et al. Association between body mass index and laboratory-confirmed influenza in middle aged and older adults: a prospective cohort study. International Journal of Obesity (2005), 2018.
- Hak E, Buskens E, van Essen GA, et al. Clinical effectiveness of influenza vaccination in persons younger than 65 years with high-risk medical conditions: the PRISMA study. Archives of Internal Medicine, 2005. 165(3): p. 274-80.
- Macintyre CR, Heywood AE, Kovoor P, et al. Ischaemic heart disease, influenza and influenza vaccination: a prospective case control study. Heart, 2013. 99(24): p. 1843-8.
- MacIntyre CR, Mahimbo A, Moa AM,Barnes M. Influenza vaccine as a coronary intervention for prevention of myocardial infarction. Heart, 2016. 102(24): p. 1953-1956.
- Mertz D, Fadel SA, Lam PP, et al. Herd effect from influenza vaccination in non-healthcare settings: a systematic review of randomised controlled trials and observational studies. Euro Surveillance, 2016. 21(42): p. pii=30378.
- Pebody R, UK Paediatric Influenza Vaccine Programme, in 3rd New Zealand Influenza Symposium. 2016: Wellington.
- Yin JK, Heywood AE, Georgousakis M, et al. Systematic review and meta-analysis of indirect protection afforded by vaccinating children against seasonal influenza: implications for policy. Clinical Infectious Diseases, 2017. 65(5): p. 719-728.
- Oshitani H, Saito R, Seki N, et al. Influenza vaccination levels and influenza-like illness in long-term-care facilities for elderly people in Niigata, Japan, during an influenza A (H3N2) epidemic. Infection Control and Hospital Epidemiology, 2000. 21(11): p. 728-30.
- Gaglani MJ, Piedra PA, Herschler GB, et al. Direct and total effectiveness of the intranasal, live-attenuated, trivalent cold-adapted influenza virus vaccine against the 2000-2001 influenza A(H1N1) and B epidemic in healthy children. Archives of Pediatrics and Adolescent Medicine, 2004. 158(1): p. 65-73.
- Ambrose CS, Yi T, Walker RE,Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatric Infectious Disease Journal, 2008. 27(8): p. 744-8.
- Australian Technical Advisory Group on Immunisation. 2018. Australian Immunisation Handbook (ed.), Canberra: Australian Government Department of Health. URL: https://immunisationhandbook.health.gov.au/ (accessed October 2019)
- Tse A, Tseng HF, Greene SK, et al. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010-2011. Vaccine, 2012. 30(11): p. 2024-31.
- Van Buynder PG, Frosst G, Van Buynder JL, et al. Increased reactions to pediatric influenza vaccination following concomitant pneumococcal vaccination. Influenza Other Respir Viruses, 2013. 7(2): p. 184-90.
- Zuckerman M, Cox R, Taylor J, et al. Rapid immune response to influenza vaccination. Lancet, 1993. 342(8879): p. 1113.
- Bednarczyk RA, Adjaye-Gbewonyo D ,Omer SB. Safety of influenza immunization during pregnancy for the fetus and the neonate. American Journal of Obstetrics and Gynecology, 2012. 207(3 Suppl): p. S38-46.
- Wang X, Li Y, O'Brien KL, et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health, 2020.
- Lee WJ, Chen LK, Tang GJ,Lan TY. The impact of influenza vaccination on hospitalizations and mortality among frail older people. Journal of the American Medical Directors Association, 2014. 15(4): p. 256-60.
- Ministry of Health. 2018 Ngā mana hauora tūtohu: Health status indicators. Ministry of Health Manatū Hauora; 2018 [updated 02 August 2018]; URL: https://www.health.govt.nz/our-work/populations/maori-health/tatau-kahukura-maori-health-statistics/nga-mana-hauora-tutohu-health-status-indicators. (accessed 25 March 2022)
- Perez-Vilar S, Wernecke M, Arya D, et al. Surveillance for Guillain-Barre syndrome after influenza vaccination among U.S. Medicare beneficiaries during the 2017-2018 season. Vaccine, 2019. 37(29): p. 3856-3865.
- Jefferson T, Di Pietrantonj C, Rivetti A, et al. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev, 2010(7): p. CD001269.
- Vellozzi C, Iqbal S ,Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clinical Infectious Diseases, 2014. 58(8): p. 1149-55.
- Stockwell MS, Broder K, LaRussa P, et al. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr, 2014. 168(3): p. 211-9.
- Australasian Society of Clinical Immunology and Allergy. 2017. Vaccination of the egg-allergic individual. ASCIA Guidelines. https://www.allergy.org.au/images/stories/pospapers/ASCIA_Guidelines_vaccination_egg_allergic_individual_2017.pdf (accessed 6 November 2019)
- Klein SL, Marriott I ,Fish EN. Sex-based differences in immune function and responses to vaccination. Transactions of the Royal Society of Tropical Medicine and Hygiene, 2015. 109(1): p. 9-15.
- AusVaxSafety. 2019 Influenza vaccine safety surveillance 2019. AusVaxSafety; 2019 [updated September 2019]; URL: https://ausvaxsafety.org.au/influenza-vaccine/2019-influenza-data. (accessed 10 May 2022)
- France EK, Glanz JM, Xu S, et al. Safety of the trivalent inactivated influenza vaccine among children: a population-based study. Archives of Pediatrics and Adolescent Medicine, 2004. 158(11): p. 1031-6.
- Kilpi T, Jokinen J, Nohynek H, et al. 2011. Reported incidence of narcolepsy in children and adolescents after Pandremix/Arepanrix vaccination. https://www.thl.fi/documents/10531/104009/Narkolepsia_posteri.pdf (accessed 20 June 2020)
- Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PloS One, 2012. 7(3): p. e33536.
- Vaarala O, Vuorela A, Partinen M, et al. Antigenic differences between AS03 adjuvanted influenza A (H1N1) pandemic vaccines: implications for pandemrix-associated narcolepsy risk. PloS One, 2014. 9(12): p. e114361.
- Dauvilliers Y, Montplaisir J, Cochen V, et al. Post-H1N1 narcolepsy-cataplexy. Sleep, 2010. 33(11): p. 1428-30.
- European Centre for Disease Prevention and Control. 2012. Narcolepsy in association with pandemic influenza vaccination (a multi-country European epidemiological investigation) (ed.), Stockholm: ECDC. URL: https://www.ecdc.europa.eu/en/publications-data/narcolepsy-association-pandemic-influenza-vaccination-multi-country-european (accessed 8 November 2019)
- Han F, Lin L, Warby SC, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Annals of Neurology, 2011. 70(3): p. 410-7.
- Sarkanen TO, Alakuijala APE, Dauvilliers YA,Partinen MM. Incidence of narcolepsy after H1N1 influenza and vaccinations: Systematic review and meta-analysis. Sleep Medicine Reviews, 2018. 38: p. 177-186.
- Butler CC, van der Velden AW, Bongard E, et al. Oseltamivir plus usual care versus usual care for influenza-like illness in primary care: an open-label, pragmatic, randomised controlled trial. Lancet, 2020. 395(10217): p. 42-52.
- Jefferson T, Jones M, Doshi P, et al. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ, 2014. 348(9 April): p. g2545.